Highly Enantioselective Iridium(I)-Catalyzed Hydrocarbonation of Alkenes: A Versatile Approach to Heterocyclic Systems Bearing Quaternary Stereocenters
- PMID: 34137152
- PMCID: PMC8456945
- DOI: 10.1002/anie.202105776
Highly Enantioselective Iridium(I)-Catalyzed Hydrocarbonation of Alkenes: A Versatile Approach to Heterocyclic Systems Bearing Quaternary Stereocenters
Abstract
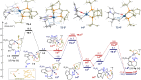
We report a versatile, highly enantioselective intramolecular hydrocarbonation reaction that provides a direct access to heteropolycyclic systems bearing chiral quaternary carbon stereocenters. The method, which relies on an iridium(I)/bisphosphine chiral catalyst, is particularly efficient for the synthesis of five-, six- and seven-membered fused indole and pyrrole products, bearing one and two stereocenters, with enantiomeric excesses of up to >99 %. DFT computational studies allowed to obtain a detailed mechanistic profile and identify a cluster of weak non-covalent interactions as key factors to control the enantioselectivity.
Keywords: C-H activation; enantioselective; heterocycles; hydrocarbonation; iridium.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- For reviews, see:
-
- Newton C. G., Wang S. G., Oliveira C. C., Cramer N., Chem. Rev. 2017, 117, 8908–8976; - PubMed
-
- Loup J., Dhawa U., Pesciaioli F., Wencel-Delord J., Ackermann L., Angew. Chem. Int. Ed. 2019, 58, 12803–12818; - PubMed
- Angew. Chem. 2019, 131, 12934–12949;
-
- Woźniak L., Tan J. F., Nguyen Q. H., Madron du Vigné A., Smal V., Cao Y. X., Cramer N., Chem. Rev. 2020, 120, 10516–10543; - PubMed
Publication types
LinkOut - more resources
Full Text Sources

