Design, Synthesis, In Vitro and In Vivo Characterization of Selective NKCC1 Inhibitors for the Treatment of Core Symptoms in Down Syndrome
- PMID: 34137257
- PMCID: PMC8311653
- DOI: 10.1021/acs.jmedchem.1c00603
Design, Synthesis, In Vitro and In Vivo Characterization of Selective NKCC1 Inhibitors for the Treatment of Core Symptoms in Down Syndrome
Abstract
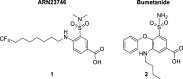
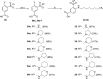
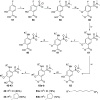
Intracellular chloride concentration [Cl-]i is defective in several neurological disorders. In neurons, [Cl-]i is mainly regulated by the action of the Na+-K+-Cl- importer NKCC1 and the K+-Cl- exporter KCC2. Recently, we have reported the discovery of ARN23746 as the lead candidate of a novel class of selective inhibitors of NKCC1. Importantly, ARN23746 is able to rescue core symptoms of Down syndrome (DS) and autism in mouse models. Here, we describe the discovery and extensive characterization of this chemical class of selective NKCC1 inhibitors, with focus on ARN23746 and other promising derivatives. In particular, we present compound 40 (ARN24092) as a backup/follow-up lead with in vivo efficacy in a mouse model of DS. These results further strengthen the potential of this new class of compounds for the treatment of core symptoms of brain disorders characterized by the defective NKCC1/KCC2 expression ratio.
Conflict of interest statement
The authors declare the following competing financial interest(s): The authors declare the following competing financial interest(s): A.C. and L.C. are named as co-inventors on the following granted patent: US 9,822,368; EP 3083959; JP 6490077; A.C. and L.C. are named as co-inventors on the patent application WO 2018/189225. A.S., M.B., A.C., M.D.V. and L.C. are named as co-inventors on patent application IT 102019000004929.
Figures

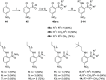
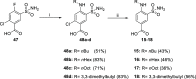
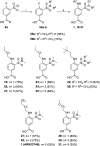
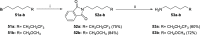
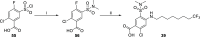
References
-
- Savardi A.; Borgogno M.; Narducci R.; La Sala G.; Ortega J. A.; Summa M.; Armirotti A.; Bertorelli R.; Contestabile A.; De Vivo M.; Cancedda L. Discovery of a Small Molecule Drug Candidate for Selective NKCC1 Inhibition in Brain Disorders. Chem 2020, 6, 2073–2096. 10.1016/j.chempr.2020.06.017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

