In Situ X-ray Absorption Spectroscopy Studies of Nanoscale Electrocatalysts
- PMID: 34138000
- PMCID: PMC7770664
- DOI: 10.1007/s40820-019-0277-x
In Situ X-ray Absorption Spectroscopy Studies of Nanoscale Electrocatalysts
Abstract
Nanoscale electrocatalysts have exhibited promising activity and stability, improving the kinetics of numerous electrochemical reactions in renewable energy systems such as electrolyzers, fuel cells, and metal-air batteries. Due to the size effect, nano particles with extreme small size have high surface areas, complicated morphology, and various surface terminations, which make them different from their bulk phases and often undergo restructuring during the reactions. These restructured materials are hard to probe by conventional ex-situ characterizations, thus leaving the true reaction centers and/or active sites difficult to determine. Nowadays, in situ techniques, particularly X-ray absorption spectroscopy (XAS), have become an important tool to obtain oxidation states, electronic structure, and local bonding environments, which are critical to investigate the electrocatalysts under real reaction conditions. In this review, we go over the basic principles of XAS and highlight recent applications of in situ XAS in studies of nanoscale electrocatalysts.
Keywords: Electrocatalyst; In situ experiments; Nanoscale; X-ray absorption spectroscopy.
Figures
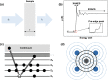


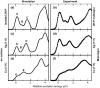


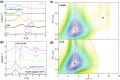

References
Publication types
LinkOut - more resources
Full Text Sources
