Antiangiogenesis-Combined Photothermal Therapy in the Second Near-Infrared Window at Laser Powers Below the Skin Tolerance Threshold
- PMID: 34138046
- PMCID: PMC7770887
- DOI: 10.1007/s40820-019-0327-4
Antiangiogenesis-Combined Photothermal Therapy in the Second Near-Infrared Window at Laser Powers Below the Skin Tolerance Threshold
Abstract
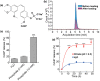
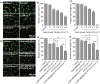
Photothermal agents with strong light absorption in the second near-infrared (NIR-II) region (1000-1350 nm) are strongly desired for successful photothermal therapy (PTT). In this work, titania-coated Au nanobipyramids (NBP@TiO2) with a strong plasmon resonance in the NIR-II window were synthesized. The NBP@TiO2 nanostructures have a high photothermal conversion efficiency of (93.3 ± 5.2)% under 1064-nm laser irradiation. They are also capable for loading an anticancer drug combretastatin A-4 phosphate (CA4P). In vitro PTT studies reveal that 1064-nm laser irradiation can efficiently ablate human lung cancer A549 cells and enhance the anticancer effect of CA4P. Moreover, the CA4P-loaded NBP@TiO2 nanostructures combined with PTT induce a synergistic antiangiogenesis effect. In vivo studies show that such CA4P-loaded NBP@TiO2 nanostructures under mild 1064-nm laser irradiation at an optical power density of 0.4 W cm-2, which is lower than the skin tolerance threshold value, exhibit a superior antitumor effect. This work presents not only the development of the NBP@TiO2 nanostructures as a novel photothermal agent responsive in the NIR-II window but also a unique combined chemo-photothermal therapy strategy for cancer therapy.
Keywords: Antiangiogenesis therapy; Core@shell nanostructures; Gold nanobipyramids; Photothermal therapy; Plasmon resonance.
Figures





References
-
- Bashkatov AN, Genina EA, Kochubey VI, Tuchin VV. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys D-Appl. Phys. 2005;38:2543–2555. doi: 10.1088/0022-3727/38/15/004. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
