Recent Progress and Approaches on Carbon-Free Energy from Water Splitting
- PMID: 34138052
- PMCID: PMC7770706
- DOI: 10.1007/s40820-019-0335-4
Recent Progress and Approaches on Carbon-Free Energy from Water Splitting
Abstract
Sunlight is the most abundant renewable energy resource, providing the earth with enough power that is capable of taking care of all of humanity's desires-a hundred times over. However, as it is at times diffuse and intermittent, it raises issues concerning how best to reap this energy and store it for times when the Sun is not shining. With increasing population in the world and modern economic development, there will be an additional increase in energy demand. Devices that use daylight to separate water into individual chemical elements may well be the answer to this issue, as water splitting produces an ideal fuel. If such devices that generate fuel were to become widely adopted, they must be low in cost, both for supplying and operation. Therefore, it is essential to research for cheap technologies for water ripping. This review summarizes the progress made toward such development, the open challenges existing, and the approaches undertaken to generate carbon-free energy through water splitting.
Keywords: Hydrogen generation; Nanostructure; Photocatalysis; Renewable energy sources; Water splitting.
Figures


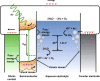
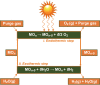
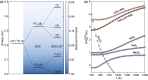
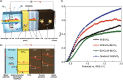

Similar articles
-
New Earth-abundant Materials for Large-scale Solar Fuels Generation.Chimia (Aarau). 2018 May 30;72(5):333-337. doi: 10.2533/chimia.2018.333. Chimia (Aarau). 2018. PMID: 29789072
-
Recent Progress in Energy-Driven Water Splitting.Adv Sci (Weinh). 2017 Jan 13;4(5):1600337. doi: 10.1002/advs.201600337. eCollection 2017 May. Adv Sci (Weinh). 2017. PMID: 28546906 Free PMC article. Review.
-
Solar fuels via artificial photosynthesis.Acc Chem Res. 2009 Dec 21;42(12):1890-8. doi: 10.1021/ar900209b. Acc Chem Res. 2009. PMID: 19902921
-
Water Splitting: From Electrode to Green Energy System.Nanomicro Lett. 2020 Jun 17;12(1):131. doi: 10.1007/s40820-020-00469-3. Nanomicro Lett. 2020. PMID: 34138146 Free PMC article. Review.
-
Directly Photoexcited Oxides for Photoelectrochemical Water Splitting.ChemSusChem. 2019 Oct 8;12(19):4337-4352. doi: 10.1002/cssc.201900849. Epub 2019 Sep 3. ChemSusChem. 2019. PMID: 31478349 Review.
Cited by
-
N-Doped Graphene-Decorated NiCo Alloy Coupled with Mesoporous NiCoMoO Nano-sheet Heterojunction for Enhanced Water Electrolysis Activity at High Current Density.Nanomicro Lett. 2021 Feb 19;13(1):77. doi: 10.1007/s40820-021-00607-5. Nanomicro Lett. 2021. PMID: 34138320 Free PMC article.
-
Three-Phase Heterojunction NiMo-Based Nano-Needle for Water Splitting at Industrial Alkaline Condition.Nanomicro Lett. 2021 Dec 9;14(1):20. doi: 10.1007/s40820-021-00744-x. Nanomicro Lett. 2021. PMID: 34882293 Free PMC article.
-
Advances in boron nitride-based nanomaterials for environmental remediation and water splitting: a review.RSC Adv. 2024 Jan 22;14(5):3447-3472. doi: 10.1039/d3ra08323c. eCollection 2024 Jan 17. RSC Adv. 2024. PMID: 38259991 Free PMC article. Review.
-
Electrochemically Grown Ultrathin Platinum Nanosheet Electrodes with Ultralow Loadings for Energy-Saving and Industrial-Level Hydrogen Evolution.Nanomicro Lett. 2023 Jun 3;15(1):144. doi: 10.1007/s40820-023-01117-2. Nanomicro Lett. 2023. PMID: 37269447 Free PMC article.
-
Synergistic Effect of Redox Dual PdO x /MnO x Cocatalysts on the Enhanced H2 Production Potential of a SnS/α-Fe2O3 Heterojunction via Ethanol Photoreforming.ACS Omega. 2022 Nov 10;7(46):42347-42358. doi: 10.1021/acsomega.2c05410. eCollection 2022 Nov 22. ACS Omega. 2022. PMID: 36440114 Free PMC article.
References
-
- Nebel R, Macounová KM, Tarábková H, Kavan L, Krtil P. Selectivity of photoelectrochemical water splitting on TiO2 anatase single crystals. J. Phys. Chem. C. 2019;123(17):10857–10867. doi: 10.1021/acs.jpcc.8b11730. - DOI
-
- Reddy CV, Reddy KR, Shetti NP, Shim J, Aminabhavi TM, Dionysiou DD. Hetero-nanostructured metal oxide-based hybrid photocatalysts for enhanced photoelectrochemical water splitting—a review. Int. J. Hydrog. Energy. 2019 doi: 10.1016/j.ijhydene.2019.02.109. - DOI
-
- Pillai S, Green MA. Plasmonics for photovoltaic applications. Solar Energy Mater. Solar Cells. 2010;94(9):1481–1486. doi: 10.1016/j.solmat.2010.02.046. - DOI
-
- Wydrzynski TJ, Hillier W, editors. Molecular Solar Fuels. London: Royal Society of Chemistry; 2011.
Publication types
LinkOut - more resources
Full Text Sources
