Metal-Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications
- PMID: 34138099
- PMCID: PMC7770922
- DOI: 10.1007/s40820-020-00423-3
Metal-Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications
Abstract
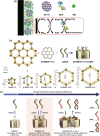
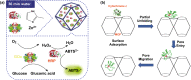
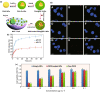
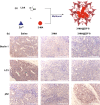
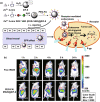
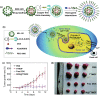
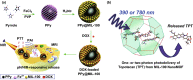
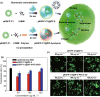
Investigation of metal-organic frameworks (MOFs) for biomedical applications has attracted much attention in recent years. MOFs are regarded as a promising class of nanocarriers for drug delivery owing to well-defined structure, ultrahigh surface area and porosity, tunable pore size, and easy chemical functionalization. In this review, the unique properties of MOFs and their advantages as nanocarriers for drug delivery in biomedical applications were discussed in the first section. Then, state-of-the-art strategies to functionalize MOFs with therapeutic agents were summarized, including surface adsorption, pore encapsulation, covalent binding, and functional molecules as building blocks. In the third section, the most recent biological applications of MOFs for intracellular delivery of drugs, proteins, and nucleic acids, especially aptamers, were presented. Finally, challenges and prospects were comprehensively discussed to provide context for future development of MOFs as efficient drug delivery systems.
Keywords: Biomedical applications; Biomolecules; Drug delivery; Drugs; Metal–organic frameworks.
Figures



References
-
- Yaghi OM, Li G, Li H. Selective binding and removal of guests in a microporous metal–organic framework. Nature. 1995;378:703–706. doi: 10.1038/378703a0. - DOI
-
- Li H, Eddaoudi M, O'Keeffe M, Yaghi OM. Design and synthesis of an exceptionally stable and highly porous metal–organic framework. Nature. 1999;402:276–279. doi: 10.1038/46248. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
