Aqueous Self-Assembly of Block Copolymers to Form Manganese Oxide-Based Polymeric Vesicles for Tumor Microenvironment-Activated Drug Delivery
- PMID: 34138110
- PMCID: PMC7770723
- DOI: 10.1007/s40820-020-00447-9
Aqueous Self-Assembly of Block Copolymers to Form Manganese Oxide-Based Polymeric Vesicles for Tumor Microenvironment-Activated Drug Delivery
Abstract
Highlights:
The formation of manganese oxide induces self-assembly of block copolymers to form polymeric vesicles.
The polymeric vesicles possessed strong stability and high drug loading capacity.
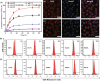
The drug-loaded polymeric vesicles have been demonstrated, especially in in vivo studies, to exhibit a higher efficacy of tumor suppression without known cardiotoxicity.
Abstract:
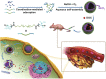
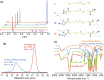
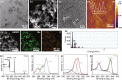
Molecular self-assembly is crucially fundamental to nature. However, the aqueous self-assembly of polymers is still a challenge. To achieve self-assembly of block copolymers [(polyacrylic acid–block–polyethylene glycol–block–polyacrylic acid (PAA68–b–PEG86–b–PAA68)] in an aqueous phase, manganese oxide (MnO2) is first generated to drive phase separation of the PAA block to form the PAA68–b–PEG86–b–PAA68/MnO2 polymeric assembly that exhibits a stable structure in a physiological medium. The polymeric assembly exhibits vesicular morphology with a diameter of approximately 30 nm and high doxorubicin (DOX) loading capacity of approximately 94%. The transformation from MnO2 to Mn2+ caused by endogenous glutathione (GSH) facilitates the disassembly of PAA68–b–PEG86–b–PAA68/MnO2 to enable its drug delivery at the tumor sites. The toxicity of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 to tumor cells has been verified in vitro and in vivo. Notably, drug-loaded polymeric vesicles have been demonstrated, especially in in vivo studies, to overcome the cardiotoxicity of DOX. We expect this work to encourage the potential application of polymer self-assembly.
Electronic supplementary material: The online version of this article (10.1007/s40820-020-00447-9) contains supplementary material, which is available to authorized users.
Keywords: Aqueous self-assembly; Drug delivery system; Polymer; Tumor microenvironment; Vesicles.
Figures

Similar articles
-
Aqueous Self-Assembly of Purely Hydrophilic Block Copolymers into Giant Vesicles.Angew Chem Int Ed Engl. 2015 Aug 10;54(33):9715-8. doi: 10.1002/anie.201502100. Epub 2015 Jul 3. Angew Chem Int Ed Engl. 2015. PMID: 26140384
-
Pharmaceutical nanotechnology: polymeric vesicles for drug and gene delivery.Expert Opin Drug Deliv. 2006 Sep;3(5):629-40. doi: 10.1517/17425247.3.5.629. Expert Opin Drug Deliv. 2006. PMID: 16948558 Review.
-
Aqueous Self-Assembly of Y-Shaped Amphiphilic Block Copolymers into Giant Vesicles.Macromol Rapid Commun. 2017 Mar;38(6). doi: 10.1002/marc.201600646. Epub 2017 Feb 6. Macromol Rapid Commun. 2017. PMID: 28166373
-
Structure, function, self-assembly, and applications of bottlebrush copolymers.Chem Soc Rev. 2015 Apr 21;44(8):2405-20. doi: 10.1039/c4cs00329b. Chem Soc Rev. 2015. PMID: 25688538
-
Polymeric vesicles: from drug carriers to nanoreactors and artificial organelles.Acc Chem Res. 2011 Oct 18;44(10):1039-49. doi: 10.1021/ar200036k. Epub 2011 May 24. Acc Chem Res. 2011. PMID: 21608994 Review.
Cited by
-
Stimuli-responsive nanocarriers for therapeutic applications in cancer.Cancer Biol Med. 2021 Mar 25;18(2):319-35. doi: 10.20892/j.issn.2095-3941.2020.0496. Cancer Biol Med. 2021. PMID: 33764711 Free PMC article. Review.
-
Ultrasound-Responsive Micelle-Encapsulated Mesenchymal Stem Cell-Derived EVs for the Treatment of Lower Limb Microcirculation Disease.ACS Omega. 2023 Dec 11;8(51):49406-49419. doi: 10.1021/acsomega.3c08133. eCollection 2023 Dec 26. ACS Omega. 2023. PMID: 38162755 Free PMC article.
-
Dissipative Particle Dynamic Simulation on Self-Assembly of Symmetric CBABC Pentablock Terpolymers in Solution.Materials (Basel). 2023 Nov 22;16(23):7273. doi: 10.3390/ma16237273. Materials (Basel). 2023. PMID: 38068017 Free PMC article.
-
Tumor microenvironment responsive nano-platform for overcoming sorafenib resistance of hepatocellular carcinoma.Mater Today Bio. 2023 Dec 15;24:100902. doi: 10.1016/j.mtbio.2023.100902. eCollection 2024 Feb. Mater Today Bio. 2023. PMID: 38188646 Free PMC article.
-
Manganese-derived biomaterials for tumor diagnosis and therapy.J Nanobiotechnology. 2024 Jun 15;22(1):335. doi: 10.1186/s12951-024-02629-8. J Nanobiotechnology. 2024. PMID: 38879519 Free PMC article.
References
-
- Newman SJ. Note on colloidal dispersions from block copolymers. Appl. Polym. Sci. 1962;6(21):S15–S16. doi: 10.1002/app.1962.070062121. - DOI
LinkOut - more resources
Full Text Sources
