Fucoidan-Based Theranostic Nanogel for Enhancing Imaging and Photodynamic Therapy of Cancer
- PMID: 34138253
- PMCID: PMC7770685
- DOI: 10.1007/s40820-020-0384-8
Fucoidan-Based Theranostic Nanogel for Enhancing Imaging and Photodynamic Therapy of Cancer
Abstract
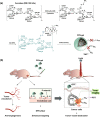
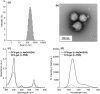
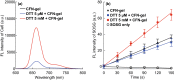
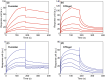
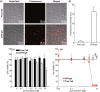
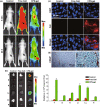
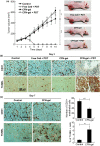
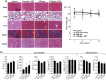
In this study, a fucoidan-based theranostic nanogel (CFN-gel) consisting of a fucoidan backbone, redox-responsive cleavable linker and photosensitizer is developed to achieve activatable near-infrared fluorescence imaging of tumor sites and an enhanced photodynamic therapy (PDT) to induce the complete death of cancer cells. A CFN-gel has nanomolar affinity for P-selectin, which is overexpressed on the surface of tumor neovascular endothelial cells as well as many other cancer cells. Therefore, a CFN-gel can enhance tumor accumulation through P-selectin targeting and the enhanced permeation and retention effect. Moreover, a CFN-gel is non-fluorescent and non-phototoxic upon its systemic administration due to the aggregation-induced self-quenching in its fluorescence and singlet oxygen generation. After internalization into cancer cells and tumor neovascular endothelial cells, its photoactivity is recovered in response to the intracellular redox potential, thereby enabling selective near-infrared fluorescence imaging and an enhanced PDT of tumors. Since a CFN-gel also shows nanomolar affinity for the vascular endothelial growth factor, it also provides a significant anti-tumor effect in the absence of light treatment in vivo. Our study indicates that a fucoidan-based theranostic nanogel is a new theranostic material for imaging and treating cancer with high efficacy and specificity.
Keywords: Activatable; Anti-angiogenic; Fucoidan; P-selectin; Theranostic nanogel.
Figures
References
-
- Fernandez JM, Bilgin MD, Grossweiner LI. Singlet oxygen generation by photodynamic agents. J. Photochem. Photobiol. B. 1997;33:131–140. doi: 10.1016/S1011-1344(96)07349-6. - DOI
-
- Bonnett R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 1995;24:19–33. doi: 10.1039/CS9952400019. - DOI
LinkOut - more resources
Full Text Sources
