Enhancing Capacitance Performance of Ti3C2Tx MXene as Electrode Materials of Supercapacitor: From Controlled Preparation to Composite Structure Construction
- PMID: 34138313
- PMCID: PMC7770793
- DOI: 10.1007/s40820-020-0415-5
Enhancing Capacitance Performance of Ti3C2Tx MXene as Electrode Materials of Supercapacitor: From Controlled Preparation to Composite Structure Construction
Abstract
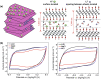
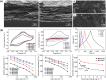
Ti3C2Tx, a novel two-dimensional layer material, is widely used as electrode materials of supercapacitor due to its good metal conductivity, redox reaction active surface, and so on. However, there are many challenges to be addressed which impede Ti3C2Tx obtaining the ideal specific capacitance, such as restacking, re-crushing, and oxidation of titanium. Recently, many advances have been proposed to enhance capacitance performance of Ti3C2Tx. In this review, recent strategies for improving specific capacitance are summarized and compared, for example, film formation, surface modification, and composite method. Furthermore, in order to comprehend the mechanism of those efforts, this review analyzes the energy storage performance in different electrolytes and influencing factors. This review is expected to predict redouble research direction of Ti3C2Tx materials in supercapacitors.
Keywords: Capacitance performance; Electrode materials; MXene; Storage mechanism; Supercapacitor; Ti3C2Tx.
Figures







Similar articles
-
Construction of Monolayer Ti3C2Tx MXene on Nickel Foam under High Electrostatic Fields for High-Performance Supercapacitors.Nanomaterials (Basel). 2024 May 19;14(10):887. doi: 10.3390/nano14100887. Nanomaterials (Basel). 2024. PMID: 38786843 Free PMC article.
-
Synergistic Charge Storage Enhancement in Supercapacitors via Ti3C2Tx MXene and CoMoO4 Nanoparticles.Micromachines (Basel). 2024 Feb 1;15(2):234. doi: 10.3390/mi15020234. Micromachines (Basel). 2024. PMID: 38398963 Free PMC article.
-
Construction of advanced zeolitic imidazolate framework derived cobalt sulfide/MXene composites as high-performance electrodes for supercapacitors.J Colloid Interface Sci. 2022 Jun;615:282-292. doi: 10.1016/j.jcis.2022.02.001. Epub 2022 Feb 2. J Colloid Interface Sci. 2022. PMID: 35144229
-
Cellulose nanofiber/MXene (Ti3C2Tx)/liquid metal film as a highly performance and flexible electrode material for supercapacitors.Int J Biol Macromol. 2024 Mar;262(Pt 2):130119. doi: 10.1016/j.ijbiomac.2024.130119. Epub 2024 Feb 10. Int J Biol Macromol. 2024. PMID: 38346617 Review.
-
Recent advances in MXene-based nanocomposites for supercapacitors.Nanotechnology. 2023 Aug 14;34(43). doi: 10.1088/1361-6528/ace8a0. Nanotechnology. 2023. PMID: 37467737 Review.
Cited by
-
Roles of Metal Ions in MXene Synthesis, Processing and Applications: A Perspective.Adv Sci (Weinh). 2022 Apr;9(12):e2200296. doi: 10.1002/advs.202200296. Epub 2022 Feb 26. Adv Sci (Weinh). 2022. PMID: 35218319 Free PMC article. Review.
-
Confinement-induced Ni-based MOF formed on Ti3C2Tx MXene support for enhanced capacitive deionization of chromium(VI).Sci Rep. 2025 Jan 29;15(1):3727. doi: 10.1038/s41598-025-87642-z. Sci Rep. 2025. PMID: 39880971 Free PMC article.
-
A Brief Review of the Role of 2D Mxene Nanosheets toward Solar Cells Efficiency Improvement.Nanomaterials (Basel). 2021 Oct 15;11(10):2732. doi: 10.3390/nano11102732. Nanomaterials (Basel). 2021. PMID: 34685175 Free PMC article. Review.
-
MXenes from MAX phases: synthesis, hybridization, and advances in supercapacitor applications.RSC Adv. 2025 Mar 24;15(12):8948-8976. doi: 10.1039/d5ra00271k. eCollection 2025 Mar 21. RSC Adv. 2025. PMID: 40129646 Free PMC article. Review.
-
MXene-Based Composites as Nanozymes in Biomedicine: A Perspective.Nanomicro Lett. 2022 Nov 4;14(1):213. doi: 10.1007/s40820-022-00958-7. Nanomicro Lett. 2022. PMID: 36333561 Free PMC article.
References
-
- Song Z, Zhou H. Towards sustainable and versatile energy storage devices: an overview of organic electrode materials. Energy Environ. Sci. 2013;6:2280–2301. doi: 10.1039/c3ee40709h. - DOI
-
- Hu H, Bai Z, Niu B, Wu M, Hua T. Binder-free bonding of modularized MXene thin films into thick film electrodes for on-chip micro-supercapacitors with enhanced areal performance metrics. J. Mater. Chem. A. 2018;6:14876–14884. doi: 10.1039/C8TA04737E. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
