Quantum monitoring of cellular metabolic activities in single mitochondria
- PMID: 34138746
- PMCID: PMC8133708
- DOI: 10.1126/sciadv.abf0573
Quantum monitoring of cellular metabolic activities in single mitochondria
Abstract
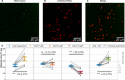
Free radicals play a vital role in all kinds of biological processes including immune responses. However, free radicals have short lifetimes and are highly reactive, making them difficult to measure using current methods. Here, we demonstrate that relaxometry measurement, or T1, inherited from the field of diamond magnetometry can be used to detect free radicals in living cells with subcellular resolution. This quantum sensing technique is based on defects in diamond, which convert a magnetic signal into an optical signal, allowing nanoscale magnetic resonance measurements. We functionalized fluorescent nanodiamonds (FNDs) to target single mitochondria within macrophage cells to detect the metabolic activity. In addition, we performed measurements on single isolated mitochondria. We were able to detect free radicals generated by individual mitochondria in either living cells or isolated mitochondria after stimulation or inhibition.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Smith R. A. J., Hartley R. C., Murphy M. P., Mitochondria-targeted small molecule therapeutics and probes. Antioxid. Redox Signal. 15, 3021–3038 (2011). - PubMed
-
- Shi J., Liu M., Shi J., Zheng G., Wang Y., Wang J., Chen Y., Lu C., Yin W., Reference gene selection for qPCR in Ammopiptanthus mongolicus under abiotic stresses and expression analysis of seven ROS-scavenging enzyme genes. Plant Cell Rep. 31, 1245–1254 (2012). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

