mTORC1 is a mechanosensor that regulates surfactant function and lung compliance during ventilator-induced lung injury
- PMID: 34138757
- PMCID: PMC8410036
- DOI: 10.1172/jci.insight.137708
mTORC1 is a mechanosensor that regulates surfactant function and lung compliance during ventilator-induced lung injury
Abstract
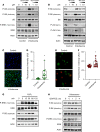
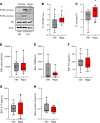
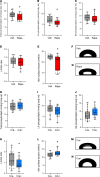
The acute respiratory distress syndrome (ARDS) is a highly lethal condition that impairs lung function and causes respiratory failure. Mechanical ventilation (MV) maintains gas exchange in patients with ARDS but exposes lung cells to physical forces that exacerbate injury. Our data demonstrate that mTOR complex 1 (mTORC1) is a mechanosensor in lung epithelial cells and that activation of this pathway during MV impairs lung function. We found that mTORC1 is activated in lung epithelial cells following volutrauma and atelectrauma in mice and humanized in vitro models of the lung microenvironment. mTORC1 is also activated in lung tissue of mechanically ventilated patients with ARDS. Deletion of Tsc2, a negative regulator of mTORC1, in epithelial cells impairs lung compliance during MV. Conversely, treatment with rapamycin at the time MV is initiated improves lung compliance without altering lung inflammation or barrier permeability. mTORC1 inhibition mitigates physiologic lung injury by preventing surfactant dysfunction during MV. Our data demonstrate that, in contrast to canonical mTORC1 activation under favorable growth conditions, activation of mTORC1 during MV exacerbates lung injury and inhibition of this pathway may be a novel therapeutic target to mitigate ventilator-induced lung injury during ARDS.
Keywords: Pulmonary surfactants; Pulmonology; Signal transduction.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

