COVID-19 Vaccination Coverage Among Pregnant Women During Pregnancy - Eight Integrated Health Care Organizations, United States, December 14, 2020-May 8, 2021
- PMID: 34138834
- PMCID: PMC8220952
- DOI: 10.15585/mmwr.mm7024e2
COVID-19 Vaccination Coverage Among Pregnant Women During Pregnancy - Eight Integrated Health Care Organizations, United States, December 14, 2020-May 8, 2021
Abstract
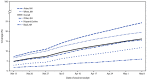
COVID-19 vaccines are critical for ending the COVID-19 pandemic; however, current data about vaccination coverage and safety in pregnant women are limited. Pregnant women are at increased risk for severe illness and death from COVID-19 compared with nonpregnant women of reproductive age, and are at risk for adverse pregnancy outcomes, such as preterm birth (1-4). Pregnant women are eligible for and can receive any of the three COVID-19 vaccines available in the United States via Emergency Use Authorization.* Data from Vaccine Safety Datalink (VSD), a collaboration between CDC and multiple integrated health systems, were analyzed to assess receipt of ≥1 dose (first or second dose of the Pfizer-BioNTech or Moderna vaccines or a single dose of the Janssen [Johnson & Johnson] vaccine) of any COVID-19 vaccine during pregnancy, receipt of first dose of a 2-dose COVID-19 vaccine (initiation), or completion of a 1- or 2-dose COVID-19 vaccination series. During December 14, 2020-May 8, 2021, a total of 135,968 pregnant women were identified, 22,197 (16.3%) of whom had received ≥1 dose of a vaccine during pregnancy. Among these 135,968 women, 7,154 (5.3%) had initiated and 15,043 (11.1%) had completed vaccination during pregnancy. Receipt of ≥1 dose of COVID-19 vaccine during pregnancy was highest among women aged 35-49 years (22.7%) and lowest among those aged 18-24 years (5.5%), and higher among non-Hispanic Asian (Asian) (24.7%) and non-Hispanic White (White) women (19.7%) than among Hispanic (11.9%) and non-Hispanic Black (Black) women (6.0%). Vaccination coverage increased among all racial and ethnic groups over the analytic period, likely because of increased eligibility for vaccination† and increased availability of vaccine over time. These findings indicate the need for improved outreach to and engagement with pregnant women, especially those from racial and ethnic minority groups who might be at higher risk for severe health outcomes because of COVID-19 (4). In addition, providing accurate and timely information about COVID-19 vaccination to health care providers, pregnant women, and women of reproductive age can improve vaccine confidence and coverage by ensuring optimal shared clinical decision-making.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. James Donahue reports grants from Janssen Global Services, LLC; Allison Naleway and Kimberly K. Vesco report grants from Pfizer. No other potential conflicts of interest were disclosed.
Figures
References
-
- Woodworth KR, Olsen EO, Neelam V, et al.; CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy—SET-NET, 16 jurisdictions, March 29–October 14, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1635–40. 10.15585/mmwr.mm6944e2 - DOI - PMC - PubMed
-
- Allotey J, Stallings E, Bonet M, et al.; for PregCOV-19 Living Systematic Review Consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. 10.1136/bmj.m3320 - DOI - PMC - PubMed
-
- Zambrano LD, Ellington S, Strid P, et al.; CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team. Update: characteristics of symptomatic women of reproductive age with laboratory–confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1641–7. 10.15585/mmwr.mm6944e3 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

