Erythrocyte adenosine A2B receptor prevents cognitive and auditory dysfunction by promoting hypoxic and metabolic reprogramming
- PMID: 34138843
- PMCID: PMC8211187
- DOI: 10.1371/journal.pbio.3001239
Erythrocyte adenosine A2B receptor prevents cognitive and auditory dysfunction by promoting hypoxic and metabolic reprogramming
Abstract
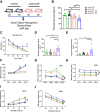
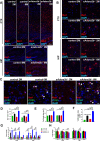
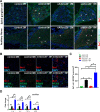
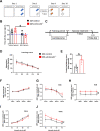
Hypoxia drives aging and promotes age-related cognition and hearing functional decline. Despite the role of erythrocytes in oxygen (O2) transport, their role in the onset of aging and age-related cognitive decline and hearing loss (HL) remains undetermined. Recent studies revealed that signaling through the erythrocyte adenosine A2B receptor (ADORA2B) promotes O2 release to counteract hypoxia at high altitude. However, nothing is known about a role for erythrocyte ADORA2B in age-related functional decline. Here, we report that loss of murine erythrocyte-specific ADORA2B (eAdora2b-/-) accelerates early onset of age-related impairments in spatial learning, memory, and hearing ability. eAdora2b-/- mice display the early aging-like cellular and molecular features including the proliferation and activation of microglia and macrophages, elevation of pro-inflammatory cytokines, and attenuation of hypoxia-induced glycolytic gene expression to counteract hypoxia in the hippocampus (HIP), cortex, or cochlea. Hypoxia sufficiently accelerates early onset of cognitive and cochlear functional decline and inflammatory response in eAdora2b-/- mice. Mechanistically, erythrocyte ADORA2B-mediated activation of AMP-activated protein kinase (AMPK) and bisphosphoglycerate mutase (BPGM) promotes hypoxic and metabolic reprogramming to enhance production of 2,3-bisphosphoglycerate (2,3-BPG), an erythrocyte-specific metabolite triggering O2 delivery. Significantly, this finding led us to further discover that murine erythroblast ADORA2B and BPGM mRNA levels and erythrocyte BPGM activity are reduced during normal aging. Overall, we determined that erythrocyte ADORA2B-BPGM axis is a key component for anti-aging and anti-age-related functional decline.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Beneficial Role of Erythrocyte Adenosine A2B Receptor-Mediated AMP-Activated Protein Kinase Activation in High-Altitude Hypoxia.Circulation. 2016 Aug 2;134(5):405-21. doi: 10.1161/CIRCULATIONAHA.116.021311. Circulation. 2016. PMID: 27482003 Free PMC article.
-
Erythrocyte Adenosine A2B Receptor-Mediated AMPK Activation: A Missing Component Counteracting CKD by Promoting Oxygen Delivery.J Am Soc Nephrol. 2019 Aug;30(8):1413-1424. doi: 10.1681/ASN.2018080862. Epub 2019 Jul 5. J Am Soc Nephrol. 2019. PMID: 31278195 Free PMC article.
-
Erythrocyte adaptive metabolic reprogramming under physiological and pathological hypoxia.Curr Opin Hematol. 2020 May;27(3):155-162. doi: 10.1097/MOH.0000000000000574. Curr Opin Hematol. 2020. PMID: 32141895 Free PMC article. Review.
-
Response to Hypoxia in Cognitive Decline.Rejuvenation Res. 2021 Aug;24(4):319-324. doi: 10.1089/rej.2021.0051. Rejuvenation Res. 2021. PMID: 34314252
-
Adenosine signaling in normal and sickle erythrocytes and beyond.Microbes Infect. 2012 Aug;14(10):863-73. doi: 10.1016/j.micinf.2012.05.005. Epub 2012 May 23. Microbes Infect. 2012. PMID: 22634345 Free PMC article. Review.
Cited by
-
Establishment of reference intervals for complete blood count in healthy adults at different altitudes on the Western Sichuan Plateau.Front Med (Lausanne). 2025 May 21;12:1586778. doi: 10.3389/fmed.2025.1586778. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40470042 Free PMC article.
-
A2B adenosine receptor signaling and regulation.Purinergic Signal. 2025 Apr;21(2):201-220. doi: 10.1007/s11302-024-10025-y. Epub 2024 Jun 4. Purinergic Signal. 2025. PMID: 38833181 Free PMC article. Review.
-
Unexplored Roles of Erythrocytes in Atherothrombotic Stroke.Neurol Int. 2023 Jan 23;15(1):124-139. doi: 10.3390/neurolint15010011. Neurol Int. 2023. PMID: 36810466 Free PMC article. Review.
-
A nomogram for predicting cancer-related cognitive impairment in lung cancer patients from a nursing science precision health model perspective.Support Care Cancer. 2025 Mar 25;33(4):320. doi: 10.1007/s00520-025-09383-z. Support Care Cancer. 2025. PMID: 40133674
-
Age-related changes after intracerebral hemorrhage: a comparative proteomics analysis of perihematomal tissue.Exp Biol Med (Maywood). 2024 Mar 25;249:10117. doi: 10.3389/ebm.2024.10117. eCollection 2024. Exp Biol Med (Maywood). 2024. PMID: 38590360 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

