Increased Subthalamic Nucleus Deep Brain Stimulation Amplitude Impairs Inhibitory Control of Eye Movements in Parkinson's Disease
- PMID: 34139037
- PMCID: PMC8759590
- DOI: 10.1111/ner.13476
Increased Subthalamic Nucleus Deep Brain Stimulation Amplitude Impairs Inhibitory Control of Eye Movements in Parkinson's Disease
Abstract
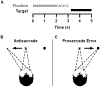
Background and objectives: Bilateral subthalamic nucleus deep brain stimulation (STN DBS) in Parkinson's disease (PD) can have detrimental effects on eye movement inhibitory control. To investigate this detrimental effect of bilateral STN DBS, we examined the effects of manipulating STN DBS amplitude on inhibitory control during the antisaccade task. The prosaccade error rate during the antisaccade task, that is, directional errors, was indicative of impaired inhibitory control. We hypothesized that as stimulation amplitude increased, the prosaccade error rate would increase.
Materials and methods: Ten participants with bilateral STN DBS completed the antisaccade task on six different stimulation amplitudes (including zero amplitude) after a 12-hour overnight withdrawal from antiparkinsonian medication.
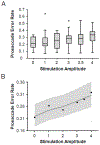
Results: We found that the prosaccade error rate increased as stimulation amplitude increased (p < 0.01). Additionally, prosaccade error rate increased as the modeled volume of tissue activated (VTA) and STN overlap decreased, but this relationship depended on stimulation amplitude (p = 0.04).
Conclusions: Our findings suggest that higher stimulation amplitude settings can be modulatory for inhibitory control. Some individual variability in the effect of stimulation amplitude can be explained by active contact location and VTA-STN overlap. Higher stimulation amplitudes are more deleterious if the active contacts fall outside of the STN resulting in a smaller VTA-STN overlap. This is clinically significant as it can inform clinical optimization of STN DBS parameters. Further studies are needed to determine stimulation amplitude effects on other aspects of cognition and whether inhibitory control deficits on the antisaccade task result in a meaningful impact on the quality of life.
Keywords: Antisaccade; Parkinson's disease; deep brain stimulation; inhibitory control; stimulation amplitude.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

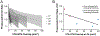

References
-
- Zauber SE, Smith P, Verhagen Metman L. Fundamentals of deep brain stimulation programming. In: Deep Brain Stimulation Management. 1st ed. Cambridge University Press; 2010:43–55.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

