Impact of dexamethasone on SARS-CoV-2 concentration kinetics and antibody response in hospitalized COVID-19 patients: results from a prospective observational study
- PMID: 34139335
- PMCID: PMC8205283
- DOI: 10.1016/j.cmi.2021.06.008
Impact of dexamethasone on SARS-CoV-2 concentration kinetics and antibody response in hospitalized COVID-19 patients: results from a prospective observational study
Abstract
Objectives: Dexamethasone has become the standard of care for severe coronavirus disease 2019 (COVID-19), but its virological impact is poorly understood. The objectives of this work were to characterize the kinetics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) concentration in the upper respiratory tract (URT) and the antibody response in patients with (D+) and without (D-) dexamethasone treatment.
Methods: Data and biosamples from hospitalized patients with severe COVID-19, enrolled between 4th March and 11th December 2020 in a prospective observational study, were analysed. SARS-CoV-2 virus concentration in serial URT samples was measured using RT-PCR. SARS-CoV-2-specific immunoglobulins A and G (IgA and IgG) were measured in serum samples using S1-ELISA.
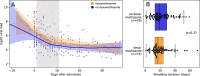
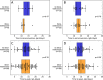
Results: We compared 101 immunocompetent patients who received dexamethasone (according to the inclusion criteria and dosage determined in the RECOVERY trial) to 93 immunocompetent patients with comparable disease severity from the first months of the pandemic, who had not been treated with dexamethasone or other glucocorticoids. We found no inter-group differences in virus concentration kinetics, duration of presence of viral loads >106 viral copies/mL (D+ median 17 days (IQR 13-24), D- 19 days (IQR 13-29)), or time from symptom onset until seroconversion (IgA: D+ median 11.5 days (IQR 11-12), D- 14 days (IQR 11.5-15.75); IgG: D+ 13 days (IQR 12-14.5), D- 12 days (IQR 11-15)).
Conclusion: Dexamethasone does not appear to lead to a change in virus clearance or a delay in antibody response in immunocompetent patients hospitalized with severe COVID-19.
Keywords: Antibody; COVID-19 nucleic acid testing; Coronavirus disease 2019 (COVID-19); Dexamethasone; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); Viral concentration.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Kurth F., Roennefarth M., Thibeault C., Corman V.M., Müller-Redetzky H., Mittermaier M. Studying the pathophysiology of coronavirus disease 2019: a protocol for the Berlin prospective COVID-19 patient cohort (Pa-COVID-19) Infection. 2020;48:619–626. doi: 10.1007/s15010-020-01464-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

