Preparing for the next pandemic: Lessons from rapid scale-up of SARS-CoV-2 testing in a South African high-throughput automated HIV molecular laboratory
- PMID: 34139371
- PMCID: PMC8205292
- DOI: 10.1016/j.ijid.2021.06.019
Preparing for the next pandemic: Lessons from rapid scale-up of SARS-CoV-2 testing in a South African high-throughput automated HIV molecular laboratory
Abstract
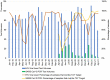
Africa's readiness to respond to the SARS-COV-2 pandemic was tested due to reliance on rapid turn-around-time of polymerase chain reaction results for clinical management, isolation and quarantine decisions. The NHLS HIV Molecular Laboratory in Johannesburg, South Africa, is one of the largest automated HIV molecular laboratories worldwide. Despite its extensive molecular capacity and experience in managing high volumes acquired from a large HIV program, significant challenges were encountered during its rapid transition to large scale SARS-CoV-2 testing. We describe the strategies employed to manage these challenges that resulted in a 30% improvement in SARS-CoV-2 test turn-around-time during the first wave peak during which approximately 25000 samples were tested per month, and further improvement during the second wave peak, with 81% within targeted turn-around-time.
Keywords: Automated; Diagnostics; Molecular; SARS-CoV-2; Scale-up.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Abbott . 2020. Abbott RealTime SARS-CoV-2 09N77-095 51-608445/R1. In.
-
- FDA . 2017. United States Food and Drug Administration. Emergency Use Authorization of medical products and related authorities: guidance for industry and other stakeholders. Retrieved from https://www.fda.gov/media/97321/download [Accessed 25 October 2020]
-
- Roche . 2020. cobas® SARS-CoV-2 Qualitative assay for use on the cobas® 6800/8800 Systems 09107991700-01EN Doc. Rev. 1.0. In.
-
- WHO . 2017. World Health Organization. PQDx_215 Protocol for the laboratory evaluation of nucleic based HIV viral load testing technologies WHO/EMP/RHT/PQT/2017.xx. Retrieved from https://www.who.int/diagnostics_laboratory/evaluations/alter/170313_prot... [Accessed 13 November 2020]
-
- Winning A. 2020. South Africa needs ‘hundreds of thousands’ of COVID-19 tests - Health Minister - CNBC Africa. https://www.cnbcafrica.com/news/2020/04/02/south-africa-needs-hundreds-o... [Accessed 13 November 2020.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

