Involvement of eNAMPT/TLR4 signaling in murine radiation pneumonitis: protection by eNAMPT neutralization
- PMID: 34139379
- PMCID: PMC8671169
- DOI: 10.1016/j.trsl.2021.06.002
Involvement of eNAMPT/TLR4 signaling in murine radiation pneumonitis: protection by eNAMPT neutralization
Abstract
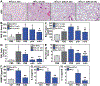
Therapeutic strategies to prevent or reduce the severity of radiation pneumonitis are a serious unmet need. We evaluated extracellular nicotinamide phosphoribosyltransferase (eNAMPT), a damage-associated molecular pattern protein (DAMP) and Toll-Like Receptor 4 (TLR4) ligand, as a therapeutic target in murine radiation pneumonitis. Radiation-induced murine and human NAMPT expression was assessed in vitro, in tissues (IHC, biochemistry, imaging), and in plasma. Wild type C57Bl6 mice (WT) and Nampt+/- heterozygous mice were exposed to 20Gy whole thoracic lung irradiation (WTLI) with or without weekly IP injection of IgG1 (control) or an eNAMPT-neutralizing polyclonal (pAb) or monoclonal antibody (mAb). BAL protein/cells and H&E staining were used to generate a WTLI severity score. Differentially-expressed genes (DEGs)/pathways were identified by RNA sequencing and bioinformatic analyses. Radiation exposure increases in vitro NAMPT expression in lung epithelium (NAMPT promoter activity) and NAMPT lung tissue expression in WTLI-exposed mice. Nampt+/- mice and eNAMPT pAb/mAb-treated mice exhibited significant histologic attenuation of WTLI-mediated lung injury with reduced levels of BAL protein and cells, and plasma levels of eNAMPT, IL-6, and IL-1β. Genomic and biochemical studies from WTLI-exposed lung tissues highlighted dysregulation of NFkB/cytokine and MAP kinase signaling pathways which were rectified by eNAMPT mAb treatment. The eNAMPT/TLR4 pathway is essentially involved in radiation pathobiology with eNAMPT neutralization an effective therapeutic strategy to reduce the severity of radiation pneumonitis.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


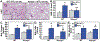


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

