Peptide receptor radionuclide therapy in patients with metastatic progressive pheochromocytoma and paraganglioma: long-term toxicity, efficacy and prognostic biomarker data of phase II clinical trials
- PMID: 34139487
- PMCID: PMC8219772
- DOI: 10.1016/j.esmoop.2021.100171
Peptide receptor radionuclide therapy in patients with metastatic progressive pheochromocytoma and paraganglioma: long-term toxicity, efficacy and prognostic biomarker data of phase II clinical trials
Abstract
Background: Pheochromocytoma and paraganglioma (PPGL) have currently only limited treatment options available for patients in the metastatic phase (mPPGL) in either post-surgery or inoperable settings. However, these rare tumors overexpress somatostatin receptors and can thus be treated with peptide receptor radionuclide therapy (PRRT). We present data about our 10-year experience treating 46 consecutive mPPGL patients with 90Y-DOTATOC or 177Lu-DOTATATE.
Patients and methods: All patients (20 men and 26 women, median age 52 years) showed positive scintigraphic imaging at 111In-octreotide or 68Ga-DOTATOC positron emission tomography/computed tomography (PET/CT). 90Y-DOTATOC was administered in 12 patients, with cumulative dosages ranging from 7.4 to 11 GBq, while 34 patients received 18.5 or 27.5GBq of 177Lu-DOTATATE. We used Southwest Oncology Group Response Evaluation Criteria in Solid Tumors criteria to evaluate treatment efficacy and Common Terminology Criteria for Adverse Events criteria to assess toxicity. The prognostic role of primary tumor site, hormone secretion, succinate dehydrogenase (SDHx) mutation, and metastatic involvement was also evaluated.
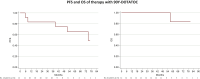
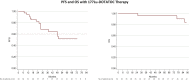
Results: Both 90Y-DOTATOC and 177Lu-DOTATATE PRRT were well tolerated by patients without significant renal or bone marrow toxicity. The median follow-up was 73 months (range 5-146 months). The overall disease control rate (DCR) was 80% [95% confidence interval (CI) 68.9% to 91.9%] with a mean five cycles of therapy. However, 177Lu-DOTATATE patients showed a longer median overall survival (mOS) than those receiving 90Y-Dotatoc and a better DCR when higher dosages were administered, even if a direct comparison was not carried out. Syndromic patients had a poorer mOS. SDHx mutations did not interfere with treatment efficacy.
Conclusions: PRRT is safe and effective for the treatment of patients with progressive mPPGL, especially at higher dosages. The longer mOS of 177Lu-DOTATATE-treated patients in our protocols indicates the former radiopharmaceutical as the better candidate for further clinical application.
Keywords: 177Lu-DOTATATE; 90Y-DOTATOC; paraganglioma; peptide receptor radionuclide therapy; phaeochromocytoma.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure The authors have declared no conflicts of interest. Data sharing The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Figures

References
-
- Lam A.K. Update on adrenal tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocr Pathol. 2017;28:213–227. - PubMed
-
- Lenders J.W., Duh Q.Y., Eisenhofer G. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–1942. - PubMed
-
- Thosani S., Ayala-Ramirez M., Roman-Gonzalez A. An overlooked, unmanaged symptom of patients with pheochromocytoma and sympathetic paraganglioma. Eur J Endocrinol. 2015;173:377–387. - PubMed
-
- Ayala-Ramirez M., Feng L., Johnson M.M. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011;96:717–725. - PubMed
-
- Lenders J.W., Eisenhofer G., Mannelli M., Pacak K. Phaechromocytoma. Lancet. 2005;366:665–675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

