Smartphone based blood pressure measurement: accuracy of the OptiBP mobile application according to the AAMI/ESH/ISO universal validation protocol
- PMID: 34139747
- PMCID: PMC8568326
- DOI: 10.1097/MBP.0000000000000556
Smartphone based blood pressure measurement: accuracy of the OptiBP mobile application according to the AAMI/ESH/ISO universal validation protocol
Abstract
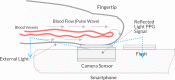
Objective: The aim of this study was to assess the accuracy of the OptiBP mobile application based on an optical signal recorded by placing the patient's fingertip on a smartphone's camera to estimate blood pressure (BP). Measurements were carried out in a general population according to existing standards of the Association for the Advancement of Medical Instrumentation (AAMI), the European Society of Hypertension (ESH) and the International Organization for Standardization (ISO).
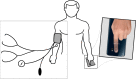
Methods: Participants were recruited during a scheduled appointment at the hypertension clinic of Lausanne University Hospital in Switzerland. Age, gender and BP distribution were collected to fulfill AAMI/ESH/ISO universal standards. Both auscultatory BP references and OptiBP were measured and compared using the opposite arm simultaneous method as described in the 81060-2:2018 ISO norm.
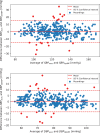
Results: A total of 353 paired recordings from 91 subjects were analyzed. For validation criterion 1, the mean ± SD between OptiBP and reference BP recordings was respectively 0.5 ± 7.7 mmHg and 0.4 ± 4.6 mmHg for SBP and DBP. For validation criterion 2, the SD of the averaged BP differences between OptiBP and reference BP per subject was 6.3 mmHg and 3.5 mmHg for SBP and DBP. OptiBP acceptance rate was 85%.
Conclusion: The smartphone embedded OptiBP cuffless mobile application fulfills the validation requirements of AAMI/ESH/ISO universal standards in a general population for the measurement of SBP and DBP.
Trial registration: ClinicalTrials.gov NCT03875248.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
M.P., G.B., A.L. and M.L. are with CSEM, the owners of the optical blood pressure monitoring technology and assignee of the related application (WO2016138965A1), of which M.P. and M.L. are inventors. U.C. and J-F.K. are working for Biospectal SA. P.S. is an advisor to Biospectal. Innosuisse - Swiss Innovation Agency, Project no. 32688.1 IP-ICT had no role in study design, data collection nor analysis, in the writing of the report nor in the decision to submit the article for publication. For the remaining authors, there are no conflicts of interest.
Figures
References
-
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392:1923–1994. - PMC - PubMed
-
- World Health Association. W.H. global status report on non-communicable diseases 2014. Vol. 2014. pp. 2. WHO Library Cataloguing; 1–281.
-
- Patel AA. Developing and evaluating mHealth solutions for chronic disease prevention in primary care. Circulation 2019; 139:392–394. - PubMed
-
- Burke LE, Ma J, Azar KM, Bennett GG, Peterson ED, Zheng Y, et al.; American Heart Association Publications Committee of the Council on Epidemiology and Prevention, Behavior Change Committee of the Council on Cardiometabolic Health, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, Council on Quality of Care and Outcomes Research, and Stroke Council. Current science on consumer use of mobile health for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation 2015; 132:1157–1213. - PMC - PubMed

