Tergitol-15-S-9 inactivates SARS-CoV-2 and boosts immunoassay signals
- PMID: 34139879
- PMCID: PMC8212844
- DOI: 10.2144/btn-2021-0044
Tergitol-15-S-9 inactivates SARS-CoV-2 and boosts immunoassay signals
Abstract
Inactivation of SARS-CoV-2 virus is necessary to mitigate risk but may interfere with diagnostic assay performance. We examined the effect of heat inactivation on a prototype SARS-CoV-2 antigen immunoassay run on the ARCHITECT automated analyzer. Recombinant full-length SARS-CoV-2 nucleocapsid protein and virus lysate detection was reduced by 66 and 31%, respectively. Several nonionic detergents were assessed as inactivation alternatives based on infectivity in cultured Vero CCL81 cells. Incubation of SARS-CoV-2 in 0.1% Tergitol 15-S-9 for 10 min significantly reduced infectivity and increased the immunoassay signal for cultured lysate and patient specimens. Tergitol 15-S-9 can inactivate SARS-CoV-2 while preserving epitopes on the nucleocapsid protein for enhanced detection by immunoassay antibodies.
Keywords: SARS-CoV-2; Tergitol 15-S-9; immunoassay; viral inactivation.
Conflict of interest statement
This study was funded by Abbott Laboratories. All authors are employees of Abbott Laboratories. A patent application has been filed for the described method. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
We acknowledge Stacey Tobin for scientific writing assistance of this manuscript.
Figures
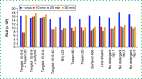
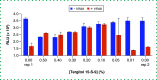
References
-
- Centers for Disease Control and Prevention. Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19) (2021). https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-biosafety-guidelines.html
-
- Centers for Disease Control and Prevention. Interim Guidelines for Collecting and Handling of Clinical Specimens for COVID-19 Testing (2021). https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
