Routine immunohistochemistry study for polyomavirus BK nephropathy in transplanted kidney biopsies, is it recommended?
- PMID: 34139999
- PMCID: PMC8212535
- DOI: 10.1186/s12882-021-02444-5
Routine immunohistochemistry study for polyomavirus BK nephropathy in transplanted kidney biopsies, is it recommended?
Abstract
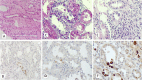
Background: Early diagnosis and treatment of Polyomavirus BK Nephropathy (PVBKN) is a challenging issue in the management of patients with kidney transplantation. Currently, histopathologic diagnosis is the gold standard method for diagnosis of PVBKN. However, typical viral inclusions may not be found in early stages of the PVBKN and should, instead, be diagnosed using immunohistochemistry (IHC) study. There is no clear consensus about routine IHC tests in the pathologic evaluation of transplanted kidney biopsy samples.
Material and methods: The current study was conducted on transplanted kidney biopsy samples, since 2016 to 2019. The patients who have presented with new onset of allograft dysfunction, at least 2 weeks after transplantation surgery, were included in our study. All these biopsy samples were evaluated with routine renal biopsy stains as well as IHC for SV40 (Simvian Virus 40) antigen. The identification of typical nuclear virus inclusion body and any nuclear positive staining on IHC (≥1+ positive result) were considered as definite evidence of PVBKN. Sensitivity, specificity, Positive Predictive and Negative Predictive Values (PPV and NPV) of histopathologic assessment without IHC study were evaluated.
Results: Among 275 included cases, 18 (6.5%) patients with PVBKN were diagnosed. In patients with PVBKN, typical viral inclusions were detected in 14 samples (77.7%), on primary histopathological examination. However, virus-infected cells were identified just after IHC study in 4 (22.2%) of patients. Sensitivity, Specifity, PPV and NPV of morphologic histopathological assay without IHC for detection of PVBKN was 77.7, 100, 100 and 98.4% respectively.
Conclusion: Routine IHC study for SV40 in all transplanted kidney biopsy samples with new onset of allograft dysfunction, will enhance the diagnostic sensitivity of early stage disease detection.
Keywords: Diagnosis; Immunohistochemistry; Polyomavirus BK nephropathy; Simvian virus 40.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures
Similar articles
-
Diagnosis of BK viral nephropathy in the renal allograft biopsy: role of fluorescence in situ hybridization.J Mol Diagn. 2012 Sep;14(5):494-500. doi: 10.1016/j.jmoldx.2012.04.004. Epub 2012 Jul 5. J Mol Diagn. 2012. PMID: 22771425
-
Testing for polyomavirus type BK DNA in plasma to identify renal-allograft recipients with viral nephropathy.N Engl J Med. 2000 May 4;342(18):1309-15. doi: 10.1056/NEJM200005043421802. N Engl J Med. 2000. PMID: 10793163
-
Silver-enhanced in situ hybridization for detection of polyomavirus DNA in patients with BK virus nephropathy.Diagn Mol Pathol. 2011 Jun;20(2):105-10. doi: 10.1097/PDM.0b013e3182015074. Diagn Mol Pathol. 2011. PMID: 21532490
-
Histologic versus molecular diagnosis of BK polyomavirus-associated nephropathy: a shifting paradigm?Clin J Am Soc Nephrol. 2006 May;1(3):374-9. doi: 10.2215/CJN.02021205. Epub 2006 Feb 8. Clin J Am Soc Nephrol. 2006. PMID: 17699234 Review.
-
Low prevalence of BK virus nephropathy on nonprotocol renal biopsies in Iranian kidney transplant recipients: one center's experience and review of the literature.Exp Clin Transplant. 2010 Dec;8(4):297-302. Exp Clin Transplant. 2010. PMID: 21143095 Review.
Cited by
-
The impact of FDA-cleared molecular solutions for BK polyomavirus quantitation.J Clin Microbiol. 2025 Mar 12;63(3):e0034824. doi: 10.1128/jcm.00348-24. Epub 2025 Jan 17. J Clin Microbiol. 2025. PMID: 39818950 Free PMC article. Review.
References
-
- Bouatou Y, Nguyen TQ, Roelofs JJ, Bemelman FJ, Michielsen L, Goldschmeding R, et al. A multicenter application of the 2018 Banff classification for BK polyomavirus-associated nephropathy in renal transplantation. Transplantation. 2019;103(12):2692–2700. doi: 10.1097/TP.0000000000002712. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

