AXL receptor tyrosine kinase: a possible therapeutic target in acute promyelocytic leukemia
- PMID: 34140003
- PMCID: PMC8210361
- DOI: 10.1186/s12885-021-08450-y
AXL receptor tyrosine kinase: a possible therapeutic target in acute promyelocytic leukemia
Abstract
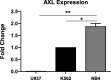
Background: Acute promyelocytic leukemia (APL) is a subset of acute myeloid leukemia (AML) which is characterized by the fusion of promyelocytic leukemia PML and retinoic acid receptor- alpha (RAR-alpha) genes. All-trans retinoic acid (ATRA) and/or arsenic trioxide (ATO) have resulted in durable cytogenetic and molecular remissions in most APL patients and have altered the natural history of the disease. Most APL patients treated with ATRA and/or ATO are now anticipated to have a nearly normal life expectancy. Unfortunately, relapse and resistance to the current treatment occur in APL patients and the outcome remains dismal in these refractory patients. AXL receptor tyrosine kinase (AXL-RTK) has been shown to increase tumour burden, provide resistance to therapy and is critical to maintain cancer stem cells (CSCs) in chronic myeloid leukemia (CML) by stabilizing β-catenin in the Wnt/β-catenin signalling pathway. However, the role of AXL-RTK has not been explored in PML/RARα-positive APL. This study aimed to explore the role of AXL-RTK receptor in PML/RARα-positive APL.
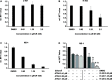
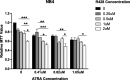
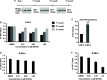
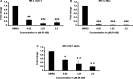
Methods and results: By using biochemical and pharmacological approaches, here we report that targeting of AXL-RTK is related to the down-regulation of β-catenin target genes including c-myc (p < 0.001), AXIN2 (p < 0.001), and HIF1α (p < 0.01) and induction of apoptosis in PML/RARα-positive APL cell line. Resistance to all-trans retinoic acid (ATRA) was also overcomed by targeting AXL-RTK with R428 in APL (p < 0.05).
Conclusion: Our results provide clear evidence of the involvement of AXL-RTK in leukemogenic potential of PML/RARα-positive APL and suggest targeting of AXL-RTK in the treatment of therapy resistant APL patients.
Keywords: AXL; Acute promyelocytic leukemia; All-trans retinoic acid; PML/RARα; Receptor tyrosine kinase; Retinoic acid receptor-α; Wnt/β-catenin pathway.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Effects of imidazole derivatives on cellular proliferation and apoptosis in myeloid leukemia.BMC Cancer. 2024 Sep 28;24(1):1200. doi: 10.1186/s12885-024-12958-4. BMC Cancer. 2024. PMID: 39342179 Free PMC article.
-
Targeting HDAC3 to overcome the resistance to ATRA or arsenic in acute promyelocytic leukemia through ubiquitination and degradation of PML-RARα.Cell Death Differ. 2023 May;30(5):1320-1333. doi: 10.1038/s41418-023-01139-8. Epub 2023 Mar 9. Cell Death Differ. 2023. PMID: 36894687 Free PMC article.
-
All-trans retinoic acid and arsenic trioxide fail to derepress the monocytic differentiation driver Irf8 in acute promyelocytic leukemia cells.Cell Death Dis. 2017 May 11;8(5):e2782. doi: 10.1038/cddis.2017.197. Cell Death Dis. 2017. PMID: 28492552 Free PMC article.
-
[Acute promyelocytic leukemia: state-of-the-art management].Rinsho Ketsueki. 2018;59(6):725-734. doi: 10.11406/rinketsu.59.725. Rinsho Ketsueki. 2018. PMID: 29973452 Review. Japanese.
-
Acute promyelocytic leukemia and variant fusion proteins: PLZF-RARα fusion protein at a glance.Semin Oncol. 2019 Apr;46(2):133-144. doi: 10.1053/j.seminoncol.2019.04.004. Epub 2019 May 7. Semin Oncol. 2019. PMID: 31126665 Review.
Cited by
-
AXL as immune regulator and therapeutic target in Acute Myeloid Leukemia: from current progress to novel strategies.Exp Hematol Oncol. 2024 Oct 4;13(1):99. doi: 10.1186/s40164-024-00566-8. Exp Hematol Oncol. 2024. PMID: 39367387 Free PMC article. Review.
-
Effects of imidazole derivatives on cellular proliferation and apoptosis in myeloid leukemia.BMC Cancer. 2024 Sep 28;24(1):1200. doi: 10.1186/s12885-024-12958-4. BMC Cancer. 2024. PMID: 39342179 Free PMC article.
-
MicroRNAs as Sensitizers of Tyrosine Kinase Inhibitor Resistance in Cancer: Small Molecule Partnerships.Pharmaceuticals (Basel). 2025 Mar 28;18(4):492. doi: 10.3390/ph18040492. Pharmaceuticals (Basel). 2025. PMID: 40283927 Free PMC article. Review.
-
Gilteritinib suppresses prostate cancer cell proliferation and migration and induces ER stress-mediated non-autophagic cytoplasmic vacuolization cell death.Med Oncol. 2025 Jun 6;42(7):241. doi: 10.1007/s12032-025-02803-4. Med Oncol. 2025. PMID: 40481367
References
-
- Seipelt G, Hofmann WK, Martin H, Wassmann B, Boehme A, Ottmann OG, Hoelzer D. Comparison of toxicity and outcome in patients with acute myeloid leukemia treated with high-dose cytosine arabinoside consolidation after induction with a regimen containing idarubicin or daunorubicin. Ann Hematol. 1998;76(3):145–151. doi: 10.1007/s002770050379. - DOI - PubMed
-
- Ben-Batalla I, Schultze A, Wroblewski M, Erdmann R, Heuser M, Waizenegger JS, Riecken K, Binder M, Schewe D, Sawall S, Witzke V, Cubas-Cordova M, Janning M, Wellbrock J, Fehse B, Hagel C, Krauter J, Ganser A, Lorens JB, Fiedler W, Carmeliet P, Pantel K, Bokemeyer C, Loges S. Axl, a prognostic and therapeutic target in acute myeloid leukemia mediates paracrine crosstalk of leukemia cells with bone marrow stroma. Blood. 2013;122(14):2443–2452. doi: 10.1182/blood-2013-03-491431. - DOI - PubMed
-
- Gallagher RE, Moser BK, Racevskis J, Poiré X, Bloomfield CD, Carroll AJ, Ketterling RP, Roulston D, Schachter-Tokarz E, Zhou DC, Chen IML, Harvey R, Koval G, Sher DA, Feusner JH, Tallman MS, Larson RA, Powell BL, Appelbaum FR, Paietta E, Willman CL, Stock W. Treatment-influenced associations of PML-RARα mutations, FLT3 mutations, and additional chromosome abnormalities in relapsed acute promyelocytic leukemia. Blood. 2012;120(10):2098–2108. doi: 10.1182/blood-2012-01-407601. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

