Neutrophils self-limit swarming to contain bacterial growth in vivo
- PMID: 34140358
- PMCID: PMC8926156
- DOI: 10.1126/science.abe7729
Neutrophils self-limit swarming to contain bacterial growth in vivo
Abstract
Neutrophils communicate with each other to form swarms in infected organs. Coordination of this population response is critical for the elimination of bacteria and fungi. Using transgenic mice, we found that neutrophils have evolved an intrinsic mechanism to self-limit swarming and avoid uncontrolled aggregation during inflammation. G protein-coupled receptor (GPCR) desensitization acts as a negative feedback control to stop migration of neutrophils when they sense high concentrations of self-secreted attractants that initially amplify swarming. Interference with this process allows neutrophils to scan larger tissue areas for microbes. Unexpectedly, this does not benefit bacterial clearance as containment of proliferating bacteria by neutrophil clusters becomes impeded. Our data reveal how autosignaling stops self-organized swarming behavior and how the finely tuned balance of neutrophil chemotaxis and arrest counteracts bacterial escape.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
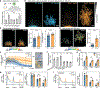




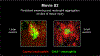
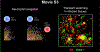
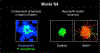
Comment in
-
Swarming motility in host defense.Science. 2021 Jun 18;372(6548):1262-1263. doi: 10.1126/science.abj3065. Science. 2021. PMID: 34140369 No abstract available.
-
Neutrophil swarm control: what goes up must come down.Signal Transduct Target Ther. 2021 Dec 6;6(1):416. doi: 10.1038/s41392-021-00836-5. Signal Transduct Target Ther. 2021. PMID: 34873155 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

