Self-Reported Medication Use and Urinary Drug Metabolites in the German Chronic Kidney Disease (GCKD) Study
- PMID: 34140400
- PMCID: PMC8729827
- DOI: 10.1681/ASN.2021010063
Self-Reported Medication Use and Urinary Drug Metabolites in the German Chronic Kidney Disease (GCKD) Study
Abstract
Background: Polypharmacy is common among patients with CKD, but little is known about the urinary excretion of many drugs and their metabolites among patients with CKD.
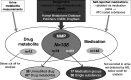
Methods: To evaluate self-reported medication use in relation to urine drug metabolite levels in a large cohort of patients with CKD, the German Chronic Kidney Disease study, we ascertained self-reported use of 158 substances and 41 medication groups, and coded active ingredients according to the Anatomical Therapeutic Chemical Classification System. We used a nontargeted mass spectrometry-based approach to quantify metabolites in urine; calculated specificity, sensitivity, and accuracy of medication use and corresponding metabolite measurements; and used multivariable regression models to evaluate associations and prescription patterns.
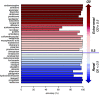
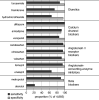
Results: Among 4885 participants, there were 108 medication-drug metabolite pairs on the basis of reported medication use and 78 drug metabolites. Accuracy was excellent for measurements of 36 individual substances in which the unchanged drug was measured in urine (median, 98.5%; range, 61.1%-100%). For 66 pairs of substances and their related drug metabolites, median measurement-based specificity and sensitivity were 99.2% (range, 84.0%-100%) and 71.7% (range, 1.2%-100%), respectively. Commonly prescribed medications for hypertension and cardiovascular risk reduction-including angiotensin II receptor blockers, calcium channel blockers, and metoprolol-showed high sensitivity and specificity. Although self-reported use of prescribed analgesics (acetaminophen, ibuprofen) was <3% each, drug metabolite levels indicated higher usage (acetaminophen, 10%-26%; ibuprofen, 10%-18%).
Conclusions: This comprehensive screen of associations between urine drug metabolite levels and self-reported medication use supports the use of pharmacometabolomics to assess medication adherence and prescription patterns in persons with CKD, and indicates under-reported use of medications available over the counter, such as analgesics.
Keywords: chronic kidney disease; medication use; pharmacometabolomics; urine metabolites.
Copyright © 2021 by the American Society of Nephrology.
Figures



References
-
- Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. ; Kidney Disease Improving Global Outcomes (KDIGO): Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 - PubMed
-
- Drawz P, Rahman M: Chronic kidney disease. Ann Intern Med 162: ITC1–ITC16, 2015 - PubMed
-
- Webster AC, Nagler EV, Morton RL, Masson P: Chronic kidney disease. Lancet 389: 1238–1252, 2017 - PubMed
-
- Lazarou J, Pomeranz BH, Corey PN: Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 279: 1200–1205, 1998 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

