Sulfiredoxin-1 attenuates injury and inflammation in acute pancreatitis through the ROS/ER stress/Cathepsin B axis
- PMID: 34140464
- PMCID: PMC8211864
- DOI: 10.1038/s41419-021-03923-1
Sulfiredoxin-1 attenuates injury and inflammation in acute pancreatitis through the ROS/ER stress/Cathepsin B axis
Abstract
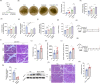
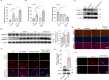
Acinar cell injury and the inflammatory response are critical bioprocesses of acute pancreatitis (AP). We investigated the role and underlying mechanism of sulfiredoxin-1 (Srxn1) in AP. Mild AP was induced by intraperitoneal injection of cerulein and severe AP was induced by partial duct ligation with cerulein stimulation or intraperitoneal injection of L-arginine in mice. Acinar cells, neutrophils, and macrophages were isolated. The pancreas was analyzed by histology, immunochemistry staining, and TUNEL assays, and the expression of certain proteins and RNAs, cytokine levels, trypsin activity, and reactive oxygen species (ROS) levels were determined. Srxn1 was inhibited by J14 or silenced by siRNA, and overexpression was introduced by a lentiviral vector. Transcriptomic analysis was used to explore the mechanism of Srxn1-mediated effects. We also evaluated the effect of adeno-associated virus (AAV)-mediated overexpression of Srxn1 by intraductal administration and the protection of AP. We found that Srxn1 expression was upregulated in mild AP but decreased in severe AP. Inhibition of Srxn1 increased ROS, histological score, the release of trypsin, and inflammatory responses in mice. Inhibition of Srxn1 expression promoted the production of ROS and induced apoptosis, while overexpression of Srxn1 led to the opposite results in acinar cells. Furthermore, inhibition of Srxn1 expression promoted the inflammatory response by accumulating and activating M1 phenotype macrophages and neutrophils in AP. Mechanistically, ROS-induced ER stress and activation of Cathepsin B, which converts trypsinogen to trypsin, were responsible for the Srxn1 inhibition-mediated effects on AP. Importantly, we demonstrated that AAV-mediated overexpression of Srxn1 attenuated AP in mice. Taken together, these results showed that Srxn1 is a protective target for AP by attenuating acinar injury and inflammation through the ROS/ER stress/Cathepsin B axis.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

