Tick extracellular vesicles enable arthropod feeding and promote distinct outcomes of bacterial infection
- PMID: 34140472
- PMCID: PMC8211691
- DOI: 10.1038/s41467-021-23900-8
Tick extracellular vesicles enable arthropod feeding and promote distinct outcomes of bacterial infection
Abstract
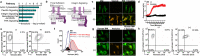
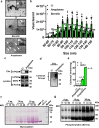
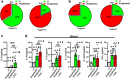
Extracellular vesicles are thought to facilitate pathogen transmission from arthropods to humans and other animals. Here, we reveal that pathogen spreading from arthropods to the mammalian host is multifaceted. Extracellular vesicles from Ixodes scapularis enable tick feeding and promote infection of the mildly virulent rickettsial agent Anaplasma phagocytophilum through the SNARE proteins Vamp33 and Synaptobrevin 2 and dendritic epidermal T cells. However, extracellular vesicles from the tick Dermacentor andersoni mitigate microbial spreading caused by the lethal pathogen Francisella tularensis. Collectively, we establish that tick extracellular vesicles foster distinct outcomes of bacterial infection and assist in vector feeding by acting on skin immunity. Thus, the biology of arthropods should be taken into consideration when developing strategies to control vector-borne diseases.
Conflict of interest statement
L.S.M. is a full-time employee of Janssen Pharmaceuticals and holds Johnson & Johnson stock. L.S.M. performed all work at his prior affiliation at Johns Hopkins University School of Medicine and he has received prior grant support from AstraZeneca, Pfizer, Boehringer Ingelheim, Regeneron Pharmaceuticals, and Moderna Therapeutics. L.S.M. was also a paid consultant for Armirall, AstraZeneca, Moderna Therapeutics and Janssen Research and Development. L.S.M. was on the scientific advisory board of Integrated Biotherapeutics and is a shareholder of Noveome Biotherapeutics. All of these aforementioned companies are developing therapeutics against infections and/or inflammatory conditions. The remaining authors declare not competing interest.
Figures




References
-
- WHO. Vector-borne Diseases (2017).
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI116620/AI/NIAID NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- R01 AI123129/AI/NIAID NIH HHS/United States
- R21 AI165520/AI/NIAID NIH HHS/United States
- R01 AI116523/AI/NIAID NIH HHS/United States
- R01 AI134696/AI/NIAID NIH HHS/United States
- R01 AI049424/AI/NIAID NIH HHS/United States
- F31 AI152215/AI/NIAID NIH HHS/United States
- R01 AR073665/AR/NIAMS NIH HHS/United States
- F31 AI138440/AI/NIAID NIH HHS/United States
- R01 AR069502/AR/NIAMS NIH HHS/United States
- R01 HL141611/HL/NHLBI NIH HHS/United States
- P01 AI138949/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

