A single cell characterisation of human embryogenesis identifies pluripotency transitions and putative anterior hypoblast centre
- PMID: 34140473
- PMCID: PMC8211662
- DOI: 10.1038/s41467-021-23758-w
A single cell characterisation of human embryogenesis identifies pluripotency transitions and putative anterior hypoblast centre
Abstract
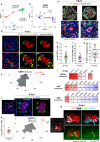
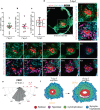
Following implantation, the human embryo undergoes major morphogenetic transformations that establish the future body plan. While the molecular events underpinning this process are established in mice, they remain unknown in humans. Here we characterise key events of human embryo morphogenesis, in the period between implantation and gastrulation, using single-cell analyses and functional studies. First, the embryonic epiblast cells transition through different pluripotent states and act as a source of FGF signals that ensure proliferation of both embryonic and extra-embryonic tissues. In a subset of embryos, we identify a group of asymmetrically positioned extra-embryonic hypoblast cells expressing inhibitors of BMP, NODAL and WNT signalling pathways. We suggest that this group of cells can act as the anterior singalling centre to pattern the epiblast. These results provide insights into pluripotency state transitions, the role of FGF signalling and the specification of anterior-posterior axis during human embryo development.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Wamaitha, S. E. & Niakan, K. K. Human pre-gastrulation development.Curr Top Dev Biol. 128, 295–338 (2018). 10.1016/bs.ctdb.2017.11.004. - PubMed
-
- Morgani, S. M. & Hadjantonakis, A.-K. Signaling regulation during gastrulation: Insights from mouse embryos and in vitro systems. Curr Top Dev Biol.137, 391–431 (2020). 10.1016/bs.ctdb.2019.11.011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

