Changes in gut microbiota in the acute phase after spinal cord injury correlate with severity of the lesion
- PMID: 34140572
- PMCID: PMC8211659
- DOI: 10.1038/s41598-021-92027-z
Changes in gut microbiota in the acute phase after spinal cord injury correlate with severity of the lesion
Abstract
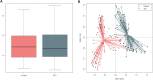
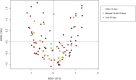
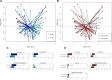
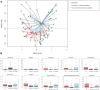
After spinal cord injury (SCI), patients face many physical and psychological issues including intestinal dysfunction and comorbidities, strongly affecting quality of life. The gut microbiota has recently been suggested to influence the course of the disease in these patients. However, to date only two studies have profiled the gut microbiota in SCI patients, months after a traumatic injury. Here we characterized the gut microbiota in a large Italian SCI population, within a short time from a not only traumatic injury. Feces were collected within the first week at the rehabilitation center (no later than 60 days after SCI), and profiled by 16S rRNA gene-based next-generation sequencing. Microbial profiles were compared to those publicly available of healthy age- and gender-matched Italians, and correlated to patient metadata, including type of SCI, spinal unit location, nutrition and concomitant antibiotic therapies. The gut microbiota of SCI patients shows distinct dysbiotic signatures, i.e. increase in potentially pathogenic, pro-inflammatory and mucus-degrading bacteria, and depletion of short-chain fatty acid producers. While robust to most host variables, such dysbiosis varies by lesion level and completeness, with the most neurologically impaired patients showing an even more unbalanced microbial profile. The SCI-related gut microbiome dysbiosis is very likely secondary to injury and closely related to the degree of completeness and severity of the lesion, regardless of etiology and time interval. This microbial layout could variously contribute to increased gut permeability and inflammation, potentially predisposing patients to the onset of severe comorbidities.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- World Health Organization. International perspectives on spinal cord injury. https://www.who.int/publications-detail/international-perspectives-on-sp.... Accessed 26 May 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

