Sunday Driver Mediates Multi-Compartment Golgi Outposts Defects Induced by Amyloid Precursor Protein
- PMID: 34140878
- PMCID: PMC8205063
- DOI: 10.3389/fnins.2021.673684
Sunday Driver Mediates Multi-Compartment Golgi Outposts Defects Induced by Amyloid Precursor Protein
Abstract
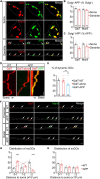
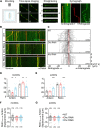
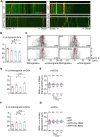
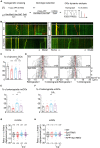
Golgi defects including Golgi fragmentation are pathological features of Alzheimer's disease (AD). As a pathogenic factor in AD, amyloid precursor protein (APP) induces Golgi fragmentation in the soma. However, how APP regulates Golgi outposts (GOs) in dendrites remains unclear. Given that APP resides in and affects the movements of GOs, and in particular, reverses the distribution of multi-compartment GOs (mcGOs), we investigated the regulatory mechanism of mcGO movements in the Drosophila larvae. Knockdown experiments showed that the bidirectional mcGO movements were cooperatively controlled by the dynein heavy chain (Dhc) and kinesin heavy chain subunits. Notably, only Dhc mediated APP's regulation of mcGO movements. Furthermore, by loss-of-function screening, the adaptor protein Sunday driver (Syd) was identified to mediate the APP-induced alteration of the direction of mcGO movements and dendritic defects. Collectively, by elucidating a model of bidirectional mcGO movements, we revealed the mechanism by which APP regulates the direction of mcGO movements. Our study therefore provides new insights into AD pathogenesis.
Keywords: Golgi outposts; Sunday driver; amyloid precursor protein; dendrite defects; motor protein.
Copyright © 2021 Du, Chang, Cheng, Zhao and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

