Lacticaseibacillus rhamnosus Reduces the Pathogenicity of Escherichia coli in Chickens
- PMID: 34140939
- PMCID: PMC8203825
- DOI: 10.3389/fmicb.2021.664604
Lacticaseibacillus rhamnosus Reduces the Pathogenicity of Escherichia coli in Chickens
Abstract
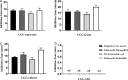
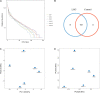
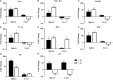
Lacticaseibacillus rhamnosus is a recognized probiotic that is widely used in scientific research and clinical applications. This study found that the Lacticaseibacillus rhamnosus GG (LGG) strain can reduce the adhesion of Escherichia coli (E. coli) to primary chicken intestinal epithelial cells by 75.7% and inhibit 41.7% of the E. coli that adhere to intestinal epithelial cells. Additionally, LGG showed strong inhibitory ability on the growth of E. coli, Staphylococcus aureus, Salmonella Paratyphi B, and Salmonella Enteritidis in vitro. Furthermore, the influence of LGG on the growth performance, intestinal flora, immunity, and disease resistance of chickens was explored. Chickens fed with LGG exhibited increased average daily weight gain and concentrations of sIgA, IgG, and IgM than did controls. After 21 days of feeding, a diet with LGG increased the diversity of intestinal microbiota and maintained intestinal health. Moreover, LGG promoted immunologic barriers by upregulating cytokines and chemokines via the Toll-like receptor. The major pro-inflammatory factors, including Myd88, NF-κB, Il6, and Il8, were upregulated compared to controls. After being challenged with E. coli, the survival rate of chickens fed with LGG was significantly higher than those in the control group, and decreased numbers of E. coli were detected in the heart and lungs of the LGG group. In summary, oral administration of LGG to chickens could improve growth performance, maintain intestinal homeostasis, and enhance innate immune response and disease resistance.
Keywords: Lacticaseibacillus rhamnosus; adhesion; disease resistance; innate immune response; intestinal microbiota.
Copyright © 2021 Guo, Zhang, Zhang, Zhang and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
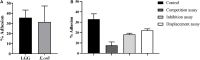



Similar articles
-
Evaluation of Lactobacillus rhamnosus GG using an Escherichia coli K88 model of piglet diarrhoea: Effects on diarrhoea incidence, faecal microflora and immune responses.Vet Microbiol. 2010 Feb 24;141(1-2):142-8. doi: 10.1016/j.vetmic.2009.09.003. Epub 2009 Sep 11. Vet Microbiol. 2010. PMID: 19782483
-
Potential probiotic Lacticaseibacillus rhamnosus MTCC-5897 attenuates Escherichia coli induced inflammatory response in intestinal cells.Arch Microbiol. 2021 Nov;203(9):5703-5713. doi: 10.1007/s00203-021-02541-x. Epub 2021 Sep 2. Arch Microbiol. 2021. PMID: 34476513
-
Dietary Administration of the Bacillus subtilis Enhances Immune Responses and Disease Resistance in Chickens.Front Microbiol. 2020 Jul 22;11:1768. doi: 10.3389/fmicb.2020.01768. eCollection 2020. Front Microbiol. 2020. PMID: 32849392 Free PMC article.
-
Molecular Mechanisms of Lacticaseibacillus rhamnosus, LGG® Probiotic Function.Microorganisms. 2024 Apr 14;12(4):794. doi: 10.3390/microorganisms12040794. Microorganisms. 2024. PMID: 38674738 Free PMC article. Review.
-
Research progress on the application of Lacticaseibacillus rhamnosus GG in pediatric respiratory diseases.Front Nutr. 2025 Feb 21;12:1553674. doi: 10.3389/fnut.2025.1553674. eCollection 2025. Front Nutr. 2025. PMID: 40062233 Free PMC article. Review.
Cited by
-
Characterization of the anti-pathogenic, genomic and phenotypic properties of a Lacticaseibacillus rhamnosus VHProbi M14 isolate.PLoS One. 2023 May 15;18(5):e0285480. doi: 10.1371/journal.pone.0285480. eCollection 2023. PLoS One. 2023. PMID: 37186610 Free PMC article.
-
Effects of dietary Lactobacillus rhamnosus GG supplementation on the production performance, egg quality, eggshell ultrastructure, and lipid metabolism of late-phase laying hens.BMC Vet Res. 2023 Sep 8;19(1):150. doi: 10.1186/s12917-023-03719-9. BMC Vet Res. 2023. PMID: 37684610 Free PMC article.
-
Butyric acid from ligilactobacillus animalis 2020MB acts on membrane BamA to control avian pathogenic escherichia coli.Poult Sci. 2025 Jun;104(6):105119. doi: 10.1016/j.psj.2025.105119. Epub 2025 Apr 1. Poult Sci. 2025. PMID: 40187014 Free PMC article.
-
First Report of Respiratory Infection Caused by Multidrug-Resistant Escherichia coli in an Ostrich in Romania.Antibiotics (Basel). 2025 Mar 31;14(4):354. doi: 10.3390/antibiotics14040354. Antibiotics (Basel). 2025. PMID: 40298517 Free PMC article.
References
-
- Abrams G. D., Bauer H., Sprinz H. (1963). Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab. Invest. 12 355–364. - PubMed
-
- Bocourt R., Savón L., Díaz J., Brizuela M. A., Elías A. (2004). Effect of the probiotic activity of Lactobacillus rhamnosus on physiological indicators of suckling pigs. Cuban J. Agric. Sci. 38 403–408.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

