Site-specific Incorporation of 2',5'-Linked Nucleic Acids Enhances Therapeutic Profile of Antisense Oligonucleotides
- PMID: 34141070
- PMCID: PMC8201499
- DOI: 10.1021/acsmedchemlett.1c00072
Site-specific Incorporation of 2',5'-Linked Nucleic Acids Enhances Therapeutic Profile of Antisense Oligonucleotides
Abstract
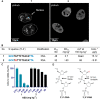
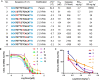
Site-specific incorporation of 2'-modifications and neutral linkages in the deoxynucleotide gap region of toxic phosphorothioate (PS) gapmer ASOs can enhance therapeutic index and safety. In this manuscript, we determined the effect of introducing 2',5'-linked RNA in the deoxynucleotide gap region on toxicity and potency of PS ASOs. Our results demonstrate that incorporation of 2',5'-linked RNA in the gap region dramatically improved hepatotoxicity profile of PS-ASOs without compromising potency and provide a novel alternate chemical approach for improving therapeutic index of ASO drugs.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Migawa M. T; Shen W.; Wan W B.; Vasquez G.; Oestergaard M. E; Low A.; De Hoyos C. L; Gupta R.; Murray S.; Tanowitz M.; Bell M.; Nichols J. G; Gaus H.; Liang X.-h.; Swayze E. E; Crooke S. T; Seth P. P Site-specific replacement of phosphorothioate with alkyl phosphonate linkages enhances the therapeutic profile of gapmer ASOs by modulating interactions with cellular proteins. Nucleic Acids Res. 2019, 47, 5465–5479. 10.1093/nar/gkz247. - DOI - PMC - PubMed
-
- Schlegel M. K.; Foster D. J.; Kel’in A. V.; Zlatev I.; Bisbe A.; Jayaraman M.; Lackey J. G.; Rajeev K. G.; Charisse K.; Harp J.; Pallan P. S.; Maier M. A.; Egli M.; Manoharan M. Chirality Dependent Potency Enhancement and Structural Impact of Glycol Nucleic Acid Modification on siRNA. J. Am. Chem. Soc. 2017, 139, 8537–8546. 10.1021/jacs.7b02694. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

