Immunomodulatory effect of dimethyloxallyl glycine/nanosilicates-loaded fibrous structure on periodontal bone remodeling
- PMID: 34141108
- PMCID: PMC8189879
- DOI: 10.1016/j.jds.2020.10.008
Immunomodulatory effect of dimethyloxallyl glycine/nanosilicates-loaded fibrous structure on periodontal bone remodeling
Abstract
Background/purpose: Relieving immuno-inflammatory responses is the prerequisite step for treating periodontitis. The angiogenic small molecule, dimethyloxalylglycine (DMOG), and osteoinductive inorganic nanomaterial, nanosilicate (nSi) have a powerful effect on bone regeneration, whereas the roles in osteoimmunomodulation have not been totally uncovered. Our study aimed to explore the immunomodulatory effect of DMOG/nSi-loaded fibrous membranes on periodontal bone remodeling.
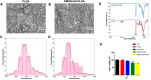
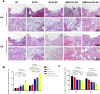
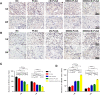
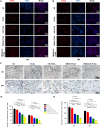
Materials and methods: The fibrous membranes were prepared by incorporating DMOG and nSi into poly (lactic-co-glycolic acid) (PLGA) with electrospinning. The morphology features, surface chemical property and biocompatibility of DMOG/nSi-PLGA fibrous membranes were characterized. Thereafter, the fibrous membranes were implanted into rat periodontal defects, bone remodeling potential and immunomodulatory effect were evaluated by micro-computed tomography (micro-CT), histological evaluation and immunohistochemical analysis.
Results: DMOG/nSi-PLGA membranes possessed favorable physicochemical properties and biocompatibility. After the fibrous membranes implanted into periodontal defects, DMOG/nSi-PLGA membranes could relieve immuno-inflammatory responses of the defects (reduction of inflammatory cell infiltration, CD40L and CD11b-positive cells), increased CD206-positive M2 macrophages, and eventually facilitated periodontal bone regeneration.
Conclusion: DMOG/nSi-PLGA fibrous membranes exert protective effects during periodontal bone defect repairing, and steer immune response towards bone regeneration. Consequently, DMOG/nSi-PLGA fibrous membranes may serve as a promising scaffold in periodontal tissue engineering.
Keywords: DMOG; Electrospinning; Immunomodulation; Nanosilicate; Periodontal bone regeneration.
© 2021 Association for Dental Sciences of the Republic of China. Publishing services by Elsevier B.V.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

Similar articles
-
Nanosilicate-functionalized nanofibrous membrane facilitated periodontal regeneration potential by harnessing periodontal ligament cell-mediated osteogenesis and immunomodulation.J Nanobiotechnology. 2023 Jul 13;21(1):223. doi: 10.1186/s12951-023-01982-4. J Nanobiotechnology. 2023. PMID: 37443072 Free PMC article.
-
Dimethyloxallyl glycine/nanosilicates-loaded osteogenic/angiogenic difunctional fibrous structure for functional periodontal tissue regeneration.Bioact Mater. 2020 Oct 26;6(4):1175-1188. doi: 10.1016/j.bioactmat.2020.10.010. eCollection 2021 Apr. Bioact Mater. 2020. PMID: 33163699 Free PMC article.
-
Synergistic effects of dimethyloxallyl glycine and recombinant human bone morphogenetic protein-2 on repair of critical-sized bone defects in rats.Sci Rep. 2017 Feb 23;7:42820. doi: 10.1038/srep42820. Sci Rep. 2017. PMID: 28230059 Free PMC article.
-
Poly(Lactic-co-Glycolic Acid): Applications and Future Prospects for Periodontal Tissue Regeneration.Polymers (Basel). 2017 Jun 1;9(6):189. doi: 10.3390/polym9060189. Polymers (Basel). 2017. PMID: 30970881 Free PMC article. Review.
-
Periodontal Tissues, Maxillary Jaw Bone, and Tooth Regeneration Approaches: From Animal Models Analyses to Clinical Applications.Nanomaterials (Basel). 2018 May 16;8(5):337. doi: 10.3390/nano8050337. Nanomaterials (Basel). 2018. PMID: 29772691 Free PMC article. Review.
Cited by
-
Electrospun Nanofibers for Periodontal Treatment: A Recent Progress.Int J Nanomedicine. 2022 Sep 12;17:4137-4162. doi: 10.2147/IJN.S370340. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36118177 Free PMC article. Review.
-
A new direction in periodontitis treatment: biomaterial-mediated macrophage immunotherapy.J Nanobiotechnology. 2024 Jun 21;22(1):359. doi: 10.1186/s12951-024-02592-4. J Nanobiotechnology. 2024. PMID: 38907216 Free PMC article. Review.
-
Nanosilicate-functionalized nanofibrous membrane facilitated periodontal regeneration potential by harnessing periodontal ligament cell-mediated osteogenesis and immunomodulation.J Nanobiotechnology. 2023 Jul 13;21(1):223. doi: 10.1186/s12951-023-01982-4. J Nanobiotechnology. 2023. PMID: 37443072 Free PMC article.
-
Vascularized bone regeneration accelerated by 3D-printed nanosilicate-functionalized polycaprolactone scaffold.Regen Biomater. 2021 Nov 12;8(6):rbab061. doi: 10.1093/rb/rbab061. eCollection 2021 Dec. Regen Biomater. 2021. PMID: 34858634 Free PMC article.
-
Functionalized multidimensional biomaterials for bone microenvironment engineering applications: Focus on osteoimmunomodulation.Front Bioeng Biotechnol. 2022 Nov 4;10:1023231. doi: 10.3389/fbioe.2022.1023231. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36406210 Free PMC article. Review.
References
-
- Woo K.M., Jung H.M., Oh J.H. Synergistic effects of dimethyloxalylglycine and butyrate incorporated into α-calcium sulfate on bone regeneration. Biomaterials. 2015;39:1–14. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

