Gut lactate-producing bacteria promote CD4 T cell recovery on Anti-retroviral therapy in HIV-infected patients
- PMID: 34141131
- PMCID: PMC8191414
- DOI: 10.1016/j.csbj.2021.05.021
Gut lactate-producing bacteria promote CD4 T cell recovery on Anti-retroviral therapy in HIV-infected patients
Abstract
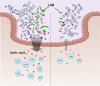
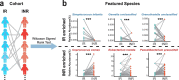
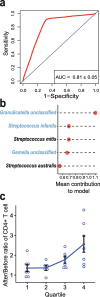
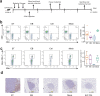
Anti-retroviral therapy (ART) effectively suppresses viral replication in HIV-infected patients, however CD4 + cell restoration to normal value is not achieved by 15-20% of patients who are called immune non-responders. Gut microbiota composition has been shown to influence host immunity. Herein, to identify intestinal microbial agents that may influence the CD4 recovery in HIV-infected patients, we utilized a "Quasi-paired cohort" method to analyze intestinal metagenome data from immunological responders (IRs) and immunological non-responders (INRs). This method identified significant enrichment for Streptococcus sp. and related lactate-producing bacteria (LAB) in IRs. In a validation cohort, positive correlations between the abundance of these LAB and the post-ART CD4 + recovery was observed, and a prediction model based on these LAB performed well in predicting immune recovery. Finally, experiments using a germ-free mouse model of antibody-induced CD4 + cell depletion showed that supplementation with a lactate-producing commensal Streptococcus thermophilus strongly promoted CD4 recovery. In conclusion, our study identified a group of LAB that was associated with enhanced immune recovery in post-ART HIV-infected patients and promotes CD4 + cell restoration in a mouse model. These findings favour supplementation of LAB commensal as a therapeutic strategy for CD4 + cell count improvement in HIV-infected patients.
Keywords: HIV; Immune recovery; Lactic acid bacteria; Metagenome.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Ghosn J., Taiwo B., Seedat S., Autran B., Katlama C. Hiv. Lancet. 2018;392(10148):685–697. - PubMed
-
- Prabhu, S., J.I. Harwell, and N. Kumarasamy, Advanced HIV: diagnosis, treatment, and prevention. Lancet HIV, 2019. 6(8): p. e540-e551. - PubMed
-
- Battegay M., Nüesch R., Hirschel B., Kaufmann G.R. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis. 2006;6(5):280–287. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
