Next-generation Bruton's tyrosine kinase inhibitor BIIB091 selectively and potently inhibits B cell and Fc receptor signaling and downstream functions in B cells and myeloid cells
- PMID: 34141433
- PMCID: PMC8204096
- DOI: 10.1002/cti2.1295
Next-generation Bruton's tyrosine kinase inhibitor BIIB091 selectively and potently inhibits B cell and Fc receptor signaling and downstream functions in B cells and myeloid cells
Abstract
Objectives: Bruton's tyrosine kinase (BTK) plays a non-redundant signaling role downstream of the B-cell receptor (BCR) in B cells and the receptors for the Fc region of immunoglobulins (FcR) in myeloid cells. Here, we characterise BIIB091, a novel, potent, selective and reversible small-molecule inhibitor of BTK.
Methods: BIIB091 was evaluated in vitro and in vivo in preclinical models and in phase 1 clinical trial.
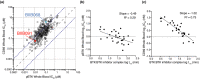
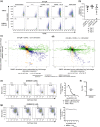
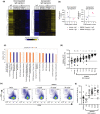
Results: In vitro, BIIB091 potently inhibited BTK-dependent proximal signaling and distal functional responses in both B cells and myeloid cells with IC50s ranging from 3 to 106 nm, including antigen presentation to T cells, a key mechanism of action thought to be underlying the efficacy of B cell-targeted therapeutics in multiple sclerosis. BIIB091 effectively sequestered tyrosine 551 in the kinase pocket by forming long-lived complexes with BTK with t 1/2 of more than 40 min, thereby preventing its phosphorylation by upstream kinases. As a key differentiating feature of BIIB091, this property explains the very potent whole blood IC50s of 87 and 106 nm observed with stimulated B cells and myeloid cells, respectively. In vivo, BIIB091 blocked B-cell activation, antibody production and germinal center differentiation. In phase 1 healthy volunteer trial, BIIB091 inhibited naïve and unswitched memory B-cell activation, with an in vivo IC50 of 55 nm and without significant impact on lymphoid or myeloid cell survival after 14 days of dosing.
Conclusion: Pharmacodynamic results obtained in preclinical and early clinical settings support the advancement of BIIB091 in phase 2 clinical trials.
Keywords: B cells; BTK inhibitor; B‐cell receptor; Fc receptor; multiple sclerosis; myeloid cells.
© 2021 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
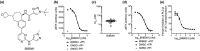


References
-
- Corneth OB, Klein Wolterink RG, Hendriks RW. BTK signaling in B cell differentiation and autoimmunity. Curr Top Microbiol Immunol 2016; 393: 67–105. - PubMed
-
- Benson MJ, Rodriguez V, von Schack D et al. Modeling the clinical phenotype of BTK inhibition in the mature murine immune system. J Immunol 2014; 193: 185–197. - PubMed
-
- Dolgin E. BTK blockers make headway in multiple sclerosis. Nat Biotechnol 2021; 39: 3–5. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
