Hemodynamic Abnormalities in the Aorta of Turner Syndrome Girls
- PMID: 34141729
- PMCID: PMC8203817
- DOI: 10.3389/fcvm.2021.670841
Hemodynamic Abnormalities in the Aorta of Turner Syndrome Girls
Abstract
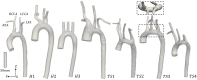
Congenital abnormalities in girls and women with Turner syndrome (TS), alongside an underlying predisposition to obesity and hypertension, contribute to an increased risk of cardiovascular disease and ultimately reduced life expectancy. We observe that children with TS present a greater variance in aortic arch morphology than their healthy counterparts, and hypothesize that their hemodynamics is also different. In this study, computational fluid dynamic (CFD) simulations were performed for four TS girls, and three age-matched healthy girls, using patient-specific inlet boundary conditions, obtained from phase-contrast MRI data. The visualization of multidirectional blood flow revealed an increase in vortical flow in the arch, supra-aortic vessels, and descending aorta, and a correlation between the presence of aortic abnormalities and disturbed flow. Compared to the relatively homogeneous pattern of time-averaged wall shear stress (TAWSS) on the healthy aortae, a highly heterogeneous distribution with elevated TAWSS values was observed in the TS geometries. Visualization of further shear stress parameters, such as oscillatory shear index (OSI), normalized relative residence time (RRTn), and transverse WSS (transWSS), revealed dissimilar heterogeneity in the oscillatory and multidirectional nature of the aortic flow. Taking into account the young age of our TS cohort (average age 13 ± 2 years) and their obesity level (75% were obese or overweight), which is believed to accelerate the initiation and progression of endothelial dysfunction, these findings may be an indication of atherosclerotic disease manifesting earlier in life in TS patients. Age, obesity and aortic morphology may, therefore, play a key role in assessing cardiovascular risk in TS children.
Keywords: Turner syndrome; atherosclerosis; cardiovascular disease; computational fluid dynamics; disturbed flow; hemodynamics; patient-specific; pediatric medicine.
Copyright © 2021 Johnston, Allen, Hall Barrientos, Mason and Kazakidi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

