(4-Aminopyridine)-PLGA-PEG as a Novel Thermosensitive and Locally Injectable Treatment for Acute Peripheral Nerve Injury
- PMID: 34142019
- PMCID: PMC8206837
- DOI: 10.1021/acsabm.0c01566
(4-Aminopyridine)-PLGA-PEG as a Novel Thermosensitive and Locally Injectable Treatment for Acute Peripheral Nerve Injury
Abstract
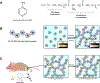
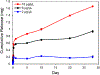
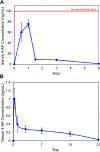
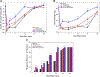
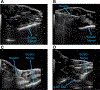
Traumatic peripheral nerve injury (TPNI) represents a major medical problem that results in loss of motor and sensory function, and in severe cases, limb paralysis and amputation. To date, there are no effective treatments beyond surgery in selective cases. In repurposing studies, we found that daily systemic administration of the FDA-approved drug 4-aminopyridine (4-AP) enhanced functional recovery after acute peripheral nerve injury. This study was aimed at constructing a novel local delivery system of 4-AP using thermogelling polymers. We optimized a thermosensitive (4-AP)-poly(lactide-co-glycolide)-b-poly(ethylene glycol)-b-poly(lactide-co-glycolide) (PLGA-PEG-PLGA) block copolymer formulation. (4-AP)-PLGA-PEG exhibited controlled release of 4-AP both in vitro and in vivo for approximately 3 weeks, with clinically relevant safe serum levels in animals. Rheological investigation showed that (4-AP)-PLGA-PEG underwent a solution to gel transition at 32 °C, a physiologically relevant temperature, allowing us to administer it to an injured limb while subsequently forming an in situ gel. A single local administration of (4-AP)-PLGA-PEG remarkably enhanced motor and sensory functional recovery on post-sciatic nerve crush injury days 1, 3, 7, 14, and 21. Moreover, immunohistochemical studies of injured nerves treated with (4-AP)-PLGA-PEG demonstrated an increased expression of neurofilament heavy chain (NF-H) and myelin protein zero (MPZ) proteins, two major markers of nerve regeneration. These findings demonstrate that (4-AP)-PLGA-PEG may be a promising long-acting local therapeutic agent in TPNI, for which no pharmacologic treatment exists.
Keywords: 4-aminopyridine; PEG; PLGA; block copolymer; crush injury; peripheral nerve; sciatic nerve; thermogel; traumatic nerve injury.
Conflict of interest statement
The authors declare the following competing financial interest(s): The senior author has an equity interest in and serves as an advisor to Peripheral Therapeutics Inc., a start-up company that may potentially benefit from the research results provided. The senior author’s ownership and role in the company have been disclosed and reviewed by The Pennsylvania State University in accordance with its conflict-of-interest policies.
Figures



References
-
- Taylor CA; Braza D; Rice JB; Dillingham T The incidence of peripheral nerve injury in extremity trauma. Am. J. Phys. Med. Rehabil 2008, 87, 381–385. - PubMed
-
- Sakuma M; Minev IR; Gribi S; Singh B; Woolf CJ; Lacour SP Chronic electrical nerve stimulation as a therapeutic intervention for peripheral nerve repair. Bioelectron Med 2015, 2, 43–48.
-
- Asplund M; Nilsson M; Jacobsson A; von Holst H Incidence of traumatic peripheral nerve injuries and amputations in Sweden between 1998 and 2006. Neuroepidemiology 2009, 32, 217–228. - PubMed
-
- Campbell WW Evaluation and management of peripheral nerve injury. Clin. Neurophysiol 2008, 119, 1951–1965. - PubMed
-
- Niver GE; Ilyas AM Management of radial nerve palsy following fractures of the humerus. Orthop. Clin. North Am 2013, 44, 419–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
