Activation of cGAS/STING pathway upon paramyxovirus infection
- PMID: 34142033
- PMCID: PMC8188492
- DOI: 10.1016/j.isci.2021.102519
Activation of cGAS/STING pathway upon paramyxovirus infection
Abstract
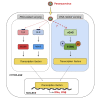
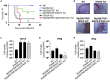
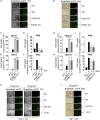
During inflammatory diseases, cancer, and infection, the cGAS/STING pathway is known to recognize foreign or self-DNA in the cytosol and activate an innate immune response. Here, we report that negative-strand RNA paramyxoviruses, Nipah virus (NiV), and measles virus (MeV), can also trigger the cGAS/STING axis. Although mice deficient for MyD88, TRIF, and MAVS still moderately control NiV infection when compared with wild-type mice, additional STING deficiency resulted in 100% lethality, suggesting synergistic roles of these pathways in host protection. Moreover, deletion of cGAS or STING resulted in decreased type I interferon production with enhanced paramyxoviral infection in both human and murine cells. Finally, the phosphorylation and ubiquitination of STING, observed during viral infections, confirmed the activation of cGAS/STING pathway by NiV and MeV. Our data suggest that cGAS/STING activation is critical in controlling paramyxovirus infection and possibly represents attractive targets to develop countermeasures against severe disease induced by these pathogens.
Keywords: Virology; immune system; molecular biology.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Adachi O., Kawai T., Takeda K., Matsumoto M., Tsutsui H., Sakagami M., Nakanishi K., Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. - PubMed
-
- Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Baccala R., Gonzalez-Quintial R., Lawson B.R., Stern M.E., Kono D.H., Beutler B., Theofilopoulos A.N. Sensors of the innate immune system: their mode of action. Nat. Rev. Rheumatol. 2009;5:448–456. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

