Efficacy and limitations of senolysis in atherosclerosis
- PMID: 34142149
- PMCID: PMC9215197
- DOI: 10.1093/cvr/cvab208
Efficacy and limitations of senolysis in atherosclerosis
Abstract
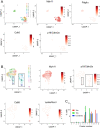
Aims: Traditional markers of cell senescence including p16, Lamin B1, and senescence-associated beta galactosidase (SAβG) suggest very high frequencies of senescent cells in atherosclerosis, while their removal via 'senolysis' has been reported to reduce atherogenesis. However, selective killing of a variety of different cell types can exacerbate atherosclerosis. We therefore examined the specificity of senescence markers in vascular smooth muscle cells (VSMCs) and the effects of genetic or pharmacological senolysis in atherosclerosis.
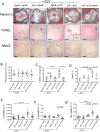
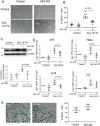
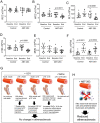
Methods and results: We examined traditional senescence markers in human and mouse VSMCs in vitro, and in mouse atherosclerosis. p16 and SAβG increased and Lamin B1 decreased in replicative senescence and stress-induced premature senescence (SIPS) of cultured human VSMCs. In contrast, mouse VSMCs undergoing SIPS showed only modest p16 up-regulation, and proliferating mouse monocyte/macrophages also expressed p16 and SAβG. Single cell RNA-sequencing (scRNA-seq) of lineage-traced mice showed increased p16 expression in VSMC-derived cells in plaques vs. normal arteries, but p16 localized to Stem cell antigen-1 (Sca1)+ or macrophage-like populations. Activation of a p16-driven suicide gene to remove p16+ vessel wall- and/or bone marrow-derived cells increased apoptotic cells, but also induced inflammation and did not change plaque size or composition. In contrast, the senolytic ABT-263 selectively reduced senescent VSMCs in culture, and markedly reduced atherogenesis. However, ABT-263 did not reduce senescence markers in vivo, and significantly reduced monocyte and platelet counts and interleukin 6 as a marker of systemic inflammation.
Conclusions: We show that genetic and pharmacological senolysis have variable effects on atherosclerosis, and may promote inflammation and non-specific effects respectively. In addition, traditional markers of cell senescence such as p16 have significant limitations to identify and remove senescent cells in atherosclerosis, suggesting that senescence studies in atherosclerosis and new senolytic drugs require more specific and lineage-restricted markers before ascribing their effects entirely to senolysis.
Keywords: Ageing; Atherosclerosis; Cell senescence.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



References
-
- Wang JC, Bennett M.. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res 2012;111:245–259. - PubMed
-
- Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Goddard M, Bennett M.. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res 2006;99:156–164. - PubMed
-
- Calvert PA, Liew T-V, Gorenne I, Clarke M, Costopoulos C, Obaid DR, O'Sullivan M, Shapiro LM, McNab DC, Densem CG, Schofield PM, Braganza D, Clarke SC, Ray KK, West NEJ, Bennett MR.. Leukocyte telomere length is associated with high-risk plaques on virtual histology intravascular ultrasound and increased pro-inflammatory activity. Arterioscler Thromb Vasc Biol 2011;31:2157–2164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RG/20/2/34763/BHF_/British Heart Foundation/United Kingdom
- RG/08/009/25841/BHF_/British Heart Foundation/United Kingdom
- RE/18/1/34212/BHF_/British Heart Foundation/United Kingdom
- RG71070/BHF_/British Heart Foundation/United Kingdom
- DH_/Department of Health/United Kingdom
- CH/2000003/12800/BHF_/British Heart Foundation/United Kingdom
- PG/14/69/31032/BHF_/British Heart Foundation/United Kingdom
- PG/16/24/32090/BHF_/British Heart Foundation/United Kingdom
- PG/16/63/32307/BHF_/British Heart Foundation/United Kingdom
- PG/16/11/32021/BHF_/British Heart Foundation/United Kingdom
- RG/13/14/30314/BHF_/British Heart Foundation/United Kingdom
- PG/13/25/30014/BHF_/British Heart Foundation/United Kingdom
- RG84554/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Research Materials

