Immunological mechanisms underlying progression of chronic wounds in recessive dystrophic epidermolysis bullosa
- PMID: 34142388
- PMCID: PMC9290674
- DOI: 10.1111/exd.14411
Immunological mechanisms underlying progression of chronic wounds in recessive dystrophic epidermolysis bullosa
Abstract
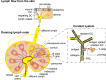
Hereditary epidermolysis bullosa (EB) is a mechanobullous skin fragility disorder characterized by defective epithelial adhesion, leading to mechanical stress-induced skin blistering. Based on the level of tissue separation within the dermal-epidermal junction, EB is categorized into simplex (EBS), junctional (JEB), dystrophic (DEB) and Kindler syndrome. There is no cure for EB, and painful chronic cutaneous wounds are one of the major complications in recessive (RDEB) patients. Although RDEB is considered a cutaneous disease, recent data support the underlying systemic immunological defects. Furthermore, chronic wounds are often colonized with pathogenic microbiota, leading to excessive inflammation and altered wound healing. Consequently, patients with RDEB suffer from a painful sensation of chronic, cutaneous itching/burning and an endless battle with bacterial infections. To improve their quality of life and life expectancy, it is important to prevent cutaneous infections, dampen chronic inflammation and stimulate wound healing. A clear scientific understanding of the immunological events underlying the maintenance of chronic poorly healing wounds in RDEB patients is necessary to improve disease management and better understand other wound healing disorders. In this review, we summarize current knowledge of the role of professional phagocytes, such as neutrophils, macrophages and dendritic cells, the role of T-cell-mediated immunity in lymphoid organs, and the association of microbiota with poor wound healing in RDEB. We conclude that RDEB patients have an underlying immunity defect that seems to affect antibacterial immunity.
Keywords: cutaneous disease; epidermolysis bullosa; immunity; inflammation; microbiota; wound healing.
© 2021 The Authors. Experimental Dermatology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- van der Kooi‐Pol MM , Duipmans JC, Jonkman MF, van Dijl JM . Host‐pathogen interactions in epidermolysis bullosa patients colonized with Staphylococcus aureus. Int J Med Microbiol. 2014;304(2):195‐203. - PubMed
-
- Has C, Nystrom A, Saeidian AH, Bruckner‐Tuderman L, Uitto J. Epidermolysis bullosa: molecular pathology of connective tissue components in the cutaneous basement membrane zone. Matrix Biol. 2018;71–72:313‐329. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

