Chemical Synthesis of TFF3 Reveals Novel Mechanistic Insights and a Gut-Stable Metabolite
- PMID: 34142550
- PMCID: PMC8273887
- DOI: 10.1021/acs.jmedchem.1c00767
Chemical Synthesis of TFF3 Reveals Novel Mechanistic Insights and a Gut-Stable Metabolite
Abstract
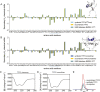
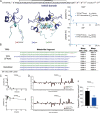
TFF3 regulates essential gastro- and neuroprotective functions, but its molecular mode of action remains poorly understood. Synthetic intractability and lack of reliable bioassays and validated receptors are bottlenecks for mechanistic and structure-activity relationship studies. Here, we report the chemical synthesis of TFF3 and its homodimer via native chemical ligation followed by oxidative folding. Correct folding was confirmed by NMR and circular dichroism, and TFF3 and its homodimer were not cytotoxic or hemolytic. TFF3, its homodimer, and the trefoil domain (TFF310-50) were susceptible to gastrointestinal degradation, revealing a gut-stable metabolite (TFF37-54; t1/2 > 24 h) that retained its trefoil structure and antiapoptotic bioactivity. We tried to validate the putative TFF3 receptors CXCR4 and LINGO2, but neither TFF3 nor its homodimer displayed any activity up to 10 μM. The discovery of a gut-stable bioactive metabolite and reliable synthetic accessibility to TFF3 and its analogues are cornerstones for future molecular probe development and structure-activity relationship studies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

