Expert opinion paper on cardiac imaging after ischemic stroke
- PMID: 34143285
- PMCID: PMC8238761
- DOI: 10.1007/s00392-021-01834-x
Expert opinion paper on cardiac imaging after ischemic stroke
Abstract
This expert opinion paper on cardiac imaging after acute ischemic stroke or transient ischemic attack (TIA) includes a statement of the "Heart and Brain" consortium of the German Cardiac Society and the German Stroke Society. The Stroke Unit-Commission of the German Stroke Society and the German Atrial Fibrillation NETwork (AFNET) endorsed this paper. Cardiac imaging is a key component of etiological work-up after stroke. Enhanced echocardiographic tools, constantly improving cardiac computer tomography (CT) as well as cardiac magnetic resonance imaging (MRI) offer comprehensive non- or less-invasive cardiac evaluation at the expense of increased costs and/or radiation exposure. Certain imaging findings usually lead to a change in medical secondary stroke prevention or may influence medical treatment. However, there is no proof from a randomized controlled trial (RCT) that the choice of the imaging method influences the prognosis of stroke patients. Summarizing present knowledge, the German Heart and Brain consortium proposes an interdisciplinary, staged standard diagnostic scheme for the detection of risk factors of cardio-embolic stroke. This expert opinion paper aims to give practical advice to physicians who are involved in stroke care. In line with the nature of an expert opinion paper, labeling of classes of recommendations is not provided, since many statements are based on expert opinion, reported case series, and clinical experience.
Keywords: Cardiac imaging; Computed tomography; Echocardiography; Expert opinion; Ischemic Stroke; Magnetic resonance imaging; Transient ischemic attack.
Conflict of interest statement
RBS has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under the grant agreement No 648131, from the European Union’s Horizon 2020 research and innovation programme under the grant agreement No 847770 (AFFECT-EU) and German Center for Cardiovascular Research (DZHK e.V.) (81Z1710103); German Ministry of Research and Education (BMBF 01ZX1408A) and ERACoSysMed3 (031L0239). RBS has received speaker honoraria and consulting fees from BMS/Pfizer outside this work. FK reports lecture/advisory board fees from Novartis, Bracco, AstraZeneca, Bayer, Alnylam, Sanofi, Shire, Pfizer, Akcea, Canon, all outside this manuscript. UB reports lecture fees/advisory board fees from Abott, Alnylam, Amgen, Astra Zeneca, Novartis outside the submitted work and travel support from Amgen, Bayer, Berlin-Chemie and Pfizer. MB reports fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Servier, Medtronic, ReCor, Vifor, Novartis and Abbott. MB is supported by the Deutsche Forschungsgemeinschaft (DFG, TTR 219, S-01). ME reports grants from Bayer and fees paid to the Charité from Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Amgen, GSK, Sanofi, Covidien, Novartis, Pfizer, all outside the submitted work. HBH reports research grants by Novartis, Medtronic, UCB Pharma and Portola Pharmaceuticals. HBH reports personal fees from Bayer AG, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, CLS Behring, UCB Pharma and Portola Pharmaceuticals. JL reports lecture fees/advisory board fees from Bayer, Boehringer Ingelheim, Bristol-Myers-Squibb, Daiichi Sankyo, Stryker and Pfizer. CM reports research cooperation with the University of Würzburg and Tomtec Imaging Systems funded by a research grant from the Bavarian Ministry of Economic Affairs, Regional Development and Energy, Germany; advisory and speakers honoraria as well as travel grants from Amgen, Tomtec, Orion Pharma, Alnylam, AKCEA, Pfizer, and EBR Systems; principal investigator in trials sponsored by Alnylam and AstraZeneca; financial support from the interdisciplinary center for clinical research—IZKF Würzburg (advanced clinician-scientist program).WP received honoraria and lecture fees from Bayer Healthcare, Pfizer, Stryker neurovascular and research grants from Boehringer Ingelheim and Stryker neurovascular. SP received speaker’s/consulting honoraria from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers-Squibb/Pizer, Daiichi Sankyo, and Werfen, reimbursement for congress traveling from Bayer, and Boehringer-Ingelheim, and research support from Bristol-Myers Squibb/Pizer, Boehringer-Ingelheim, Daiichi Sankyo, and Helena Laboratories (all outside of the present work). TR received consulting honoraria, speakers’ honoraria and travel support from Bristol-Myers Squibb/Pfizer, Boehringer-Ingelheim, Bayer HealthCare and DaichiiSankyo, outside the submitted work. AR reports lecture honoraria from Pfizer, Boehringer Ingelheim, Bayer, BMS, Berlin Chemie. JR reports lecture fees/advisory board fees from Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Amgen, Pfizer (unrelated to the submitted manuscript). WRS reports research grant Health Economic Research Zentrum, Ferrer. Speakers’ Bureau: Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo Co., Bayer, Pfizer, Medtronic, Ferrer as well as consultant/advisory board fees from Boehringer Ingelheim, Daiichi Sankyo Co., Medtronic. GT reports study grants by Bayer, lecture fees/advisory board fees from Acandis, Bayer, Boehringer Ingelheim, Bristol-Myers-Squibb / Pfizer, Daiichi Sankyo, and Stryker. RW reports grants from Bundesministerium für Bildung und Forschung (BMBF), Deutsches Zentrum für Herz-/Kreislaufforschung, Deutsche Forschungsgemeinschaft, European Union and Medtronic, all outside the submitted work. He received personal fees from AstraZeneca, Bayer, Berlin Chemie, Boehringer Ingelheim, Bristol-Myers-Squibb, CVRX, Daiichi Sankyo, Gilead, Medtronic Novartis, Pfizer, Pharmacosmos, Servier, outside the submitted work. KGH reports study grants by Bayer and Sanofi-Aventis, lecture fees/advisory board fees from Abbott, AstraZeneca, Bayer, Biotronik, Boehringer Ingelheim, Bristol-Myers-Squibb, Daiichi Sankyo, Edwards Lifesciences, Medtronic, Pfizer, Premier Research and Sanofi-Aventis. The other authors report no conflict of interest. Sollte formal vor den COIs von KGH aufgeführt werden.
Figures

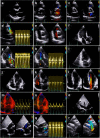

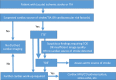
References
-
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/str.0000000000000211. - DOI - PubMed
-
- Haeusler KG, Groschel K, Kohrmann M, Anker SD, Brachmann J, Bohm M, Diener HC, Doehner W, Endres M, Gerloff C, Huttner HB, Kaps M, Kirchhof P, Nabavi DG, Nolte CH, Pfeilschifter W, Pieske B, Poli S, Schabitz WR, Thomalla G, Veltkamp R, Steiner T, Laufs U, Rother J, Wachter R, Schnabel R. Expert opinion paper on atrial fibrillation detection after ischemic stroke. Clin Res Cardiol. 2018;107(10):871–880. doi: 10.1007/s00392-018-1256-9. - DOI - PubMed
-
- Holmes M, Rathbone J, Littlewood C, Rawdin A, Stevenson M, Stevens J, Archer R, Evans P, Wang J. Routine echocardiography in the management of stroke and transient ischaemic attack: a systematic review and economic evaluation. Health Technol Assess (Winchester, England) 2014;18(16):1–176. doi: 10.3310/hta18160. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

