SARS-CoV-2-Specific Antibody and T Cell Response Kinetics According to Symptom Severity
- PMID: 34143752
- PMCID: PMC8437179
- DOI: 10.4269/ajtmh.20-1594
SARS-CoV-2-Specific Antibody and T Cell Response Kinetics According to Symptom Severity
Abstract
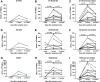
Data on the longevity of humoral and cell-mediated immune responses against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in patients with coronavirus disease 2019 (COVID-19) are limited. We evaluated the detailed kinetics of antibody and T-cell responses at the acute, convalescent, and post-convalescent phases in COVID-19 patients with a wide range of severity. We enrolled patients with COVID-19 prospectively from four hospitals and one community treatment center between February 2020 and January 2021. symptom severity was classified as mild, moderate, or severe/critical. Patient blood samples were collected at 1 week (acute), 1 month (convalescent), and 2 months after symptom onset (post-convalescent). Human SARS-CoV-2 IgG and IgM antibodies were measured using in-house-developed ELISA. The SARS-CoV-2-specific T-cell responses against overlapping peptides of spike proteins and nucleoprotein were measured by interferon-γ enzyme-linked immunospot assays. Twenty-five COVID-19 patients were analyzed (mild, n = 5; moderate, n = 9; severe/critical, n = 11). IgM and IgG antibody responses peaked at 1 month after symptom onset and decreased at 2 months. IgG response levels were significantly greater in the severe/critical group compared with other groups. Interferon-γ-producing T-cell responses increased between 1 week and 1 month after symptom onset, and had a trend toward decreasing at 2 months, but did not show significant differences according to severity. Our data indicate that SARS-CoV-2-specific antibody responses were greater in those with severe symptoms and waned after reaching a peak around 1 month after symptom onset. However, SARS-CoV-2-specific T-cell responses were not significantly different according to symptom severity, and decreased slowly during the post-convalescent phase.
Figures

References
-
- Long QX et al.2020. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26: 845–848. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

