Mepolizumab targets multiple immune cells in aspirin-exacerbated respiratory disease
- PMID: 34144111
- PMCID: PMC9096876
- DOI: 10.1016/j.jaci.2021.05.043
Mepolizumab targets multiple immune cells in aspirin-exacerbated respiratory disease
Abstract
Background: Eosinophilic asthma and nasal polyposis are hallmarks of aspirin-exacerbated respiratory disease (AERD), and IL-5 inhibition has been shown to provide therapeutic benefit. However, IL-5Rα is expressed on many cells in addition to eosinophils, and the mechanisms by which IL-5 inhibition leads to clinical benefit in eosinophilic asthma and nasal polyposis are unlikely to be due exclusively to antieosinophil effects.
Objective: We sought to identify the mechanisms by which anti-IL-5 treatment with mepolizumab improves respiratory inflammation in AERD.
Methods: The clinical characteristics, circulating granulocytes, nasal scraping transcripts, eosinophilic cationic protein, tryptase, and antibody levels, and urinary and nasal eicosanoid levels were measured for 18 subjects with AERD who were taking mepolizumab and compared with those of 18 matched subjects with AERD who were not taking mepolizumab.
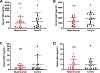
Results: Subjects taking mepolizumab had significantly fewer peripheral blood eosinophils and basophils, and those cells that remained had higher surface CRTH2 expression than did the cells from subjects not taking mepolizumab. Nasal prostaglandin F2α, prostaglandin D2 metabolites, leukotriene B4, and thromboxane levels were lower in subjects taking mepolizumab, as were urinary levels of tetranor-prostaglandin D2 and leukotriene E4. The nasal epithelial cell transcripts that were overexpressed among subjects with AERD who were taking mepolizumab were enriched for genes involved in tight junction formation and cilium organization. Nasal and urinary prostaglandin E2, tryptase, and antibody levels were not different between the 2 groups.
Conclusion: IL-5 inhibition in AERD decreases production of inflammatory eicosanoids and upregulates tight junction-associated nasal epithelial cell transcripts, likely due to decreased IL-5 signaling on tissue mast cells, eosinophils, and epithelial cells. These direct effects on multiple relevant immune cells contribute to the mechanism of benefit afforded by mepolizumab.
Keywords: Aspirin-exacerbated respiratory disease; CRTH2; IL-5; chronic rhinosinusitis; leukotriene; mepolizumab; nasal polyp; prostaglandin D(2); prostaglandin F(2α).
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Conflict of Interest: T Laidlaw has served on scientific advisory boards for GlaxoSmithKline and Sanofi-Genzyme, Optinose, and Regeneron. K Buchheit has served on scientific advisory boards for AstraZeneca and GlaxoSmithKline. J Bensko has served on scientific advisory boards for GlaxoSmithKline. J Ordovas-Montanes reports compensation for consulting services with Cellarity and Hovione. A Shalek reports compensation for consulting and/or SAB membership from Merck, Honeycomb Biotechnologies, Cellarity, Repertoire Immune Medicines, Hovione, Third Rock Ventures, Ochre Bio, Relation Therapeutics, and Dahlia Biosciences. A Shalek has received research support from Merck, Novartis, Leo Pharma, Janssen, the Bill and Melinda Gates Foundation, the Moore Foundation, the Pew-Stewart Trust, Fondation MIT, the Chan Zuckerberg Initiative, Novo Nordisk and the FDA unrelated to this work. The rest of the authors have no conflicts of interest to disclose.
Figures




References
-
- Bhattacharyya N Assessing the additional disease burden of polyps in chronic rhinosinusitis. Ann Otol Rhinol Laryngol. 2009;118(3):185–9. - PubMed
-
- Campbell AP, Phillips KM, Hoehle LP, Feng AL, Bergmark RW, Caradonna DS, et al. Depression symptoms and lost productivity in chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2017;118(3):286–9. - PubMed
-
- McMains KC, Kountakis SE. Medical and surgical considerations in patients with Samter’s triad. American journal of rhinology. 2006;20(6):573–6. - PubMed
-
- Laidlaw TM, Prussin C, Panettieri RA, Lee S, Ferguson BJ, Adappa ND, et al. Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size. Laryngoscope. 2019;129(2):E61–E6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

