Autoantibodies against ACE2 and angiotensin type-1 receptors increase severity of COVID-19
- PMID: 34144328
- PMCID: PMC8193025
- DOI: 10.1016/j.jaut.2021.102683
Autoantibodies against ACE2 and angiotensin type-1 receptors increase severity of COVID-19
Abstract
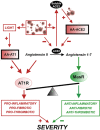
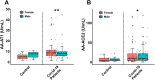
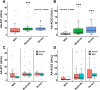
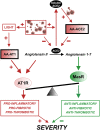
The renin-angiotensin system (RAS) plays a major role in COVID-19. Severity of several inflammation-related diseases has been associated with autoantibodies against RAS, particularly agonistic autoantibodies for angiotensin type-1 receptors (AA-AT1) and autoantibodies against ACE2 (AA-ACE2). Disease severity of COVID-19 patients was defined as mild, moderate or severe following the WHO Clinical Progression Scale and determined at medical discharge. Serum AA-AT1 and AA-ACE2 were measured in COVID-19 patients (n = 119) and non-infected controls (n = 23) using specific solid-phase, sandwich enzyme-linked immunosorbent assays. Serum LIGHT (TNFSF14; tumor necrosis factor ligand superfamily member 14) levels were measured with the corresponding assay kit. At diagnosis, AA-AT1 and AA-ACE2 levels were significantly higher in the COVID-19 group relative to controls, and we observed significant association between disease outcome and serum AA-AT1 and AA-ACE2 levels. Mild disease patients had significantly lower levels of AA-AT1 (p < 0.01) and AA-ACE2 (p < 0.001) than moderate and severe patients. No significant differences were detected between males and females. The increase in autoantibodies was not related to comorbidities potentially affecting COVID-19 severity. There was significant positive correlation between serum levels of AA-AT1 and LIGHT (TNFSF14; rPearson = 0.70, p < 0.001). Both AA-AT1 (by agonistic stimulation of AT1 receptors) and AA-ACE2 (by reducing conversion of Angiotensin II into Angiotensin 1-7) may lead to increase in AT1 receptor activity, enhance proinflammatory responses and severity of COVID-19 outcome. Patients with high levels of autoantibodies require more cautious control after diagnosis. Additionally, the results encourage further studies on the possible protective treatment with AT1 receptor blockers in COVID-19.
Keywords: Autoantibody; Autoimmunity; LIGHT; Outcome prediction; Renin-angiotensin system; SARS-CoV-2.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Serum angiotensin type-1 receptor autoantibodies and neurofilament light chain as markers of neuroaxonal damage in post-COVID patients.Front Immunol. 2025 Apr 22;16:1571027. doi: 10.3389/fimmu.2025.1571027. eCollection 2025. Front Immunol. 2025. PMID: 40330487 Free PMC article.
-
Development of ACE2 autoantibodies after SARS-CoV-2 infection.PLoS One. 2021 Sep 3;16(9):e0257016. doi: 10.1371/journal.pone.0257016. eCollection 2021. PLoS One. 2021. PMID: 34478478 Free PMC article.
-
AT1 receptor autoantibodies mediate effects of metabolic syndrome on dopaminergic vulnerability.Brain Behav Immun. 2023 Feb;108:255-268. doi: 10.1016/j.bbi.2022.12.009. Epub 2022 Dec 16. Brain Behav Immun. 2023. PMID: 36535607
-
Downregulation of Membrane-bound Angiotensin Converting Enzyme 2 (ACE2) Receptor has a Pivotal Role in COVID-19 Immunopathology.Curr Drug Targets. 2021;22(3):254-281. doi: 10.2174/1389450121666201020154033. Curr Drug Targets. 2021. PMID: 33081670 Review.
-
Sex differences in COVID-19: candidate pathways, genetics of ACE2, and sex hormones.Am J Physiol Heart Circ Physiol. 2021 Jan 1;320(1):H296-H304. doi: 10.1152/ajpheart.00755.2020. Epub 2020 Dec 4. Am J Physiol Heart Circ Physiol. 2021. PMID: 33275517 Free PMC article. Review.
Cited by
-
Drugs Modulating Renin-Angiotensin System in COVID-19 Treatment.Biomedicines. 2022 Feb 21;10(2):502. doi: 10.3390/biomedicines10020502. Biomedicines. 2022. PMID: 35203711 Free PMC article. Review.
-
Animals Experimentally Infected with SARS-CoV-2 Generate Functional Autoantibodies against G-Protein-Coupled Receptors.Biomedicines. 2023 Sep 28;11(10):2668. doi: 10.3390/biomedicines11102668. Biomedicines. 2023. PMID: 37893042 Free PMC article.
-
Age and genotype dependent erythropoietin protection in COVID-19.World J Stem Cells. 2021 Oct 26;13(10):1513-1529. doi: 10.4252/wjsc.v13.i10.1513. World J Stem Cells. 2021. PMID: 34786155 Free PMC article. Review.
-
Preeclampsia in the Context of COVID-19: Mechanisms, Pathophysiology, and Clinical Outcomes.Am J Reprod Immunol. 2024 Aug;92(2):e13915. doi: 10.1111/aji.13915. Am J Reprod Immunol. 2024. PMID: 39132825 Free PMC article. Review.
-
Angiotensin II receptor I auto-antibodies following SARS-CoV-2 infection.PLoS One. 2021 Nov 17;16(11):e0259902. doi: 10.1371/journal.pone.0259902. eCollection 2021. PLoS One. 2021. PMID: 34788328 Free PMC article.
References
-
- Cohen J.B., Hanff T.C., William P., Sweitzer N., Rosado-Santander N.R., Medina C., Rodriguez-Mori J.E., Renna N., Chang T.I., Corrales-Medina V., Andrade-Villanueva J.F., Barbagelata A., Cristodulo-Cortez R., Diaz-Cucho O.A., Spaak J., Alfonso C.E., Valdivia-Vega R., Villavicencio-Carranza M., Ayala-Garcia R.J., Castro-Callirgos C.A., Gonzalez-Hernandez L.A., Bernales-Salas E.F., Coacalla-Guerra J.C., Salinas-Herrera C.D., Nicolosi L., Basconcel M., Byrd J.B., Sharkoski T., Bendezu-Huasasquiche L.E., Chittams J., Edmonston D.L., Vasquez C.R., Chirinos J.A. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir. Med. 2021;9:275–284. doi: 10.1016/S2213-2600(20)30558-0. - DOI - PMC - PubMed
-
- Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

