Production, purification and availability of 211At: Near term steps towards global access
- PMID: 34144505
- PMCID: PMC8448941
- DOI: 10.1016/j.nucmedbio.2021.05.007
Production, purification and availability of 211At: Near term steps towards global access
Abstract
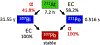
The promising characteristics of the 7.2-h radiohalogen 211At have long been recognized; including having chemical properties suitable for labeling targeting vectors ranging from small organic molecules to proteins, and the emission of only one α-particle per decay, providing greater control over off-target effects. Unfortunately, the impact of 211At within the targeted α-particle therapy domain has been constrained by its limited availability. Paradoxically, the most commonly used production method - via the 209Bi(α,2n)211At reaction - utilizes a widely available natural material (bismuth) as the target and straightforward cyclotron irradiation methodology. On the other hand, the most significant impediment to widespread 211At availability is the need for an accelerator capable of generating ≥28 MeV α-particles with sufficient beam intensities to make clinically relevant levels of 211At. In this review, current methodologies for the production and purification of 211At - both by the direct production route noted above and via a 211Rn generator system - will be discussed. The capabilities of cyclotrons that currently produce 211At will be summarized and the characteristics of other accelerators that could be utilized for this purpose will be described. Finally, the logistics of networks, both academic and commercial, for facilitating 211At distribution to locations remote from production sites will be addressed.
Keywords: Alpha emitter; Astatine; Cyclotron; Radionuclide production; Targeted alpha-particle therapy.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest M.R.Z. is a co-inventor on patents and patent applications for high-level (211)At labeling as well as (211)At-labeled compounds of potential utility for targeted radiotherapy. The authors declare no other known potential competing interests.
Figures







References
-
- Johnson E, Turkington T, Jaszczak R, Gilland D, Vaidyanathan G, Greer K, et al.Quantitation of 211At in small volumes for evaluation of targeted radiotherapy in animal models. Nucl Med Biol 1995;22:45–54. - PubMed
-
- Palm S, Humm JL, Rundqvist R, and Jacobsson L. Microdosimetry of Astatine‐211 single‐cell irradiation: Role of daughter Polonium‐211 diffusion. Med. Phys 2004;31:218–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

