Activation of mTORC1 at late endosomes misdirects T cell fate decision in older individuals
- PMID: 34145066
- PMCID: PMC8422387
- DOI: 10.1126/sciimmunol.abg0791
Activation of mTORC1 at late endosomes misdirects T cell fate decision in older individuals
Abstract
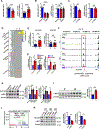
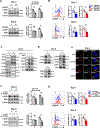
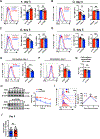
The nutrient-sensing mammalian target of rapamycin (mTOR) is integral to cell fate decisions after T cell activation. Sustained mTORC1 activity favors the generation of terminally differentiated effector T cells instead of follicular helper and memory T cells. This is particularly pertinent for T cell responses of older adults who have sustained mTORC1 activation despite dysfunctional lysosomes. Here, we show that lysosome-deficient T cells rely on late endosomes rather than lysosomes as an mTORC1 activation platform, where mTORC1 is activated by sensing cytosolic amino acids. T cells from older adults have an increased expression of the plasma membrane leucine transporter SLC7A5 to provide a cytosolic amino acid source. Hence, SLC7A5 and VPS39 deficiency (a member of the HOPS complex promoting early to late endosome conversion) substantially reduced mTORC1 activities in T cells from older but not young individuals. Late endosomal mTORC1 is independent of the negative-feedback loop involving mTORC1-induced inactivation of the transcription factor TFEB that controls expression of lysosomal genes. The resulting sustained mTORC1 activation impaired lysosome function and prevented lysosomal degradation of PD-1 in CD4+ T cells from older adults, thereby inhibiting their proliferative responses. VPS39 silencing of human T cells improved their expansion to pertussis and to SARS-CoV-2 peptides in vitro. Furthermore, adoptive transfer of CD4+ Vps39-deficient LCMV-specific SMARTA cells improved germinal center responses, CD8+ memory T cell generation, and recall responses to infection. Thus, curtailing late endosomal mTORC1 activity is a promising strategy to enhance T cell immunity.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures




References
-
- Nikolich-Zugich J, The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol 19, 10–19 (2018). - PubMed
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K, Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289, 179–186 (2003). - PubMed
-
- Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A, Network C-LI, Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA, (2020). - PMC - PubMed
-
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062 (2020). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

