Combination of 131I-trastuzumab and lanatoside C enhanced therapeutic efficacy in HER2 positive tumor model
- PMID: 34145369
- PMCID: PMC8213814
- DOI: 10.1038/s41598-021-92460-0
Combination of 131I-trastuzumab and lanatoside C enhanced therapeutic efficacy in HER2 positive tumor model
Abstract
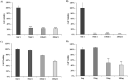
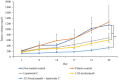
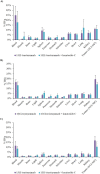
Lanatoside C has a promising anti-tumor activity and is a potential candidate for radiosensitizers. In this study, we have investigated the therapeutic efficacy of the combination of 131I-trastuzumab and lanatoside C for inhibition of human epidermal growth factor receptor 2 (HER2) positive tumor progression in NCI-N87 xenograft model. The combination treatment (131I-trastuzumab and lanatoside C) showed highest cytotoxicity when compared to non-treated control or trastuzumab alone or 131I alone or 131I-trastuzumab alone in vitro. Biodistribution studies using 131I-trastuzumab or combination of 131I-trastuzumab and lanatoside C showed tumor uptake in BALB/c nude mice bearing HER2 positive NCI-N87 tumor xenograft model. The higher tumor uptake was observed in 131I-trastuzumab (19.40 ± 0.04% ID/g) than in the combination of 131I-trastuzumab and lanatoside C (14.02 ± 0.02% ID/g) at 24 h post-injection. Most importantly, an antitumor effect was observed in mice that received the combination of 131I-trastuzumab and lanatoside C (p = 0.009) when compared to control. In addition, mice received lanatoside C alone (p = 0.085) or 131I-trastuzumab alone (p = 0.160) did not significantly inhibit tumor progression compared with control. Taken together, our data suggest that combination of 131I-trastuzumab and lanatoside C might be a potential synergistic treatment for radioimmunotherapy to control the HER2 positive tumor.
Conflict of interest statement
The authors declare no competing interests.
Figures
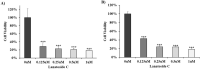
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

