Timed daily exercise remodels circadian rhythms in mice
- PMID: 34145388
- PMCID: PMC8213798
- DOI: 10.1038/s42003-021-02239-2
Timed daily exercise remodels circadian rhythms in mice
Abstract
Regular exercise is important for physical and mental health. An underexplored and intriguing property of exercise is its actions on the body's 24 h or circadian rhythms. Molecular clock cells in the brain's suprachiasmatic nuclei (SCN) use electrical and chemical signals to orchestrate their activity and convey time of day information to the rest of the brain and body. To date, the long-lasting effects of regular physical exercise on SCN clock cell coordination and communication remain unresolved. Utilizing mouse models in which SCN intercellular neuropeptide signaling is impaired as well as those with intact SCN neurochemical signaling, we examined how daily scheduled voluntary exercise (SVE) influenced behavioral rhythms and SCN molecular and neuronal activities. We show that in mice with disrupted neuropeptide signaling, SVE promotes SCN clock cell synchrony and robust 24 h rhythms in behavior. Interestingly, in both intact and neuropeptide signaling deficient animals, SVE reduces SCN neural activity and alters GABAergic signaling. These findings illustrate the potential utility of regular exercise as a long-lasting and effective non-invasive intervention in the elderly or mentally ill where circadian rhythms can be blunted and poorly aligned to the external world.
Conflict of interest statement
The authors declare no competing interests.
Figures
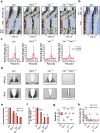


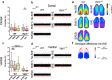

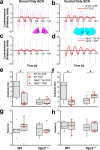
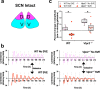
References
Publication types
MeSH terms
Substances
Grants and funding
- BB/J003441/1 /BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/M02329X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/R019223/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 107851/Z/15/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources

