Intestinal Flora is a Key Factor in Insulin Resistance and Contributes to the Development of Polycystic Ovary Syndrome
- PMID: 34145455
- PMCID: PMC8375444
- DOI: 10.1210/endocr/bqab118
Intestinal Flora is a Key Factor in Insulin Resistance and Contributes to the Development of Polycystic Ovary Syndrome
Abstract
Context: The key gut microbial biomarkers for polycystic ovarian syndrome (PCOS) and how dysbiosis causes insulin resistance and PCOS remain unclear.
Objective: To assess the characteristics of intestinal flora in PCOS and explore whether abnormal intestinal flora can affect insulin resistance and promote PCOS and whether chenodeoxycholic acid (CDCA) can activate intestinal farnesoid X receptor (FXR), improving glucose metabolism in PCOS.
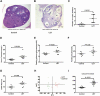
Setting and design: The intestinal flora of treatment-naïve PCOS patients and hormonally healthy controls was analyzed. Phenotype analysis, intestinal flora analysis, and global metabolomic profiling of caecal contents were performed on a letrozole-induced PCOS mouse model; similar analyses were conducted after 35 days of antibiotic treatment on the PCOS mouse model, and glucose tolerance testing was performed on the PCOS mouse model after a 35-day CDCA treatment. Mice receiving fecal microbiota transplants from PCOS patients or healthy controls were evaluated after 10 weeks.
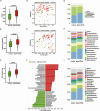
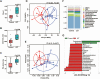
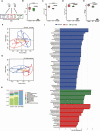
Results: Bacteroides was significantly enriched in treatment-naïve PCOS patients. The enrichment in Bacteroides was reproduced in the PCOS mouse model. Gut microbiota removal ameliorated the PCOS phenotype and insulin resistance and increased relative FXR mRNA levels in the ileum and serum fibroblast growth factor 15 levels. PCOS stool-transplanted mice exhibited insulin resistance at 10 weeks but not PCOS. Treating the PCOS mouse model with CDCA improved glucose metabolism.
Conclusions: Bacteroides is a key microbial biomarker in PCOS and shows diagnostic value. Gut dysbiosis can cause insulin resistance. FXR activation might play a beneficial rather than detrimental role in glucose metabolism in PCOS.
Keywords: FXR; PCOS; insulin resistance; intestinal flora.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society.
Figures



Comment in
-
Key role for gut microbiota in PCOS development.Nat Rev Endocrinol. 2021 Sep;17(9):515. doi: 10.1038/s41574-021-00534-w. Nat Rev Endocrinol. 2021. PMID: 34194010 No abstract available.
References
-
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270-284. - PubMed
-
- Jacewicz-Święcka M, Kowalska I. Polycystic ovary syndrome and the risk of cardiometabolic complications in longitudinal studies. Diabetes Metab Res Rev. 2018;34(8):e3054. - PubMed
-
- Bajuk Studen K, Jensterle Sever M, Pfeifer M. Cardiovascular risk and subclinical cardiovascular disease in polycystic ovary syndrome. Front Horm Res. 2013;40:64-82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

