COVID-19-related neuropathology and microglial activation in elderly with and without dementia
- PMID: 34145669
- PMCID: PMC8412067
- DOI: 10.1111/bpa.12997
COVID-19-related neuropathology and microglial activation in elderly with and without dementia
Abstract
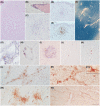
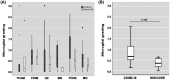
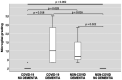
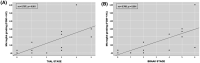
The actual role of SARS-CoV-2 in brain damage remains controversial due to lack of matched controls. We aim to highlight to what extent is neuropathology determined by SARS-CoV-2 or by pre-existing conditions. Findings of 9 Coronavirus disease 2019 (COVID-19) cases and 6 matched non-COVID controls (mean age 79 y/o) were compared. Brains were analyzed through immunohistochemistry to detect SARS-CoV-2, lymphocytes, astrocytes, endothelium, and microglia. A semi-quantitative scoring was applied to grade microglial activation. Thal-Braak stages and the presence of small vessel disease were determined in all cases. COVID-19 cases had a relatively short clinical course (0-32 days; mean: 10 days), and did not undergo mechanical ventilation. Five patients with neurocognitive disorder had delirium. All COVID-19 cases showed non-SARS-CoV-2-specific changes including hypoxic-agonal alterations, and a variable degree of neurodegeneration and/or pre-existent SVD. The neuroinflammatory picture was dominated by ameboid CD68 positive microglia, while only scant lymphocytic presence and very few traces of SARS-CoV-2 were detected. Microglial activation in the brainstem was significantly greater in COVID-19 cases (p = 0.046). Instead, microglial hyperactivation in the frontal cortex and hippocampus was clearly associated to AD pathology (p = 0.001), regardless of the SARS-CoV-2 infection. In COVID-19 cases complicated by delirium (all with neurocognitive disorders), there was a significant enhancement of microglia in the hippocampus (p = 0.048). Although higher in cases with both Alzheimer's pathology and COVID-19, cortical neuroinflammation is not related to COVID-19 per se but mostly to pre-existing neurodegeneration. COVID-19 brains seem to manifest a boosting of innate immunity with microglial reinforcement, and adaptive immunity suppression with low number of brain lymphocytes probably related to systemic lymphopenia. Thus, no neuropathological evidence of SARS-CoV-2-specific encephalitis is detectable. The microglial hyperactivation in the brainstem, and in the hippocampus of COVID-19 patients with delirium, appears as a specific topographical phenomenon, and probably represents the neuropathological basis of the "COVID-19 encephalopathic syndrome" in the elderly.
Keywords: COVID-19; dementia; elderly; microglia; neurocognitive disorders; neuropathology.
© 2021 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital.Brain. 2021 Oct 22;144(9):2696-2708. doi: 10.1093/brain/awab148. Brain. 2021. PMID: 33856027 Free PMC article.
-
Neuropathology of patients with COVID-19 in Germany: a post-mortem case series.Lancet Neurol. 2020 Nov;19(11):919-929. doi: 10.1016/S1474-4422(20)30308-2. Epub 2020 Oct 5. Lancet Neurol. 2020. PMID: 33031735 Free PMC article.
-
COVID-19 Pathology in the Lung, Kidney, Heart and Brain: The Different Roles of T-Cells, Macrophages, and Microthrombosis.Cells. 2022 Oct 4;11(19):3124. doi: 10.3390/cells11193124. Cells. 2022. PMID: 36231087 Free PMC article.
-
Invited Review: The spectrum of neuropathology in COVID-19.Neuropathol Appl Neurobiol. 2021 Feb;47(1):3-16. doi: 10.1111/nan.12667. Epub 2020 Oct 20. Neuropathol Appl Neurobiol. 2021. PMID: 32935873 Review.
-
SARS-CoV-2 and the brain: A review of the current knowledge on neuropathology in COVID-19.Brain Pathol. 2021 Nov;31(6):e13013. doi: 10.1111/bpa.13013. Epub 2021 Aug 13. Brain Pathol. 2021. PMID: 34390282 Free PMC article.
Cited by
-
SARS-CoV-2 drives NLRP3 inflammasome activation in human microglia through spike protein.Mol Psychiatry. 2023 Jul;28(7):2878-2893. doi: 10.1038/s41380-022-01831-0. Epub 2022 Nov 1. Mol Psychiatry. 2023. PMID: 36316366 Free PMC article.
-
The neurobiology of SARS-CoV-2 infection.Nat Rev Neurosci. 2024 Jan;25(1):30-42. doi: 10.1038/s41583-023-00769-8. Epub 2023 Dec 4. Nat Rev Neurosci. 2024. PMID: 38049610 Review.
-
Brain autopsies of critically ill COVID-19 patients demonstrate heterogeneous profile of acute vascular injury, inflammation and age-linked chronic brain diseases.Acta Neuropathol Commun. 2022 Dec 17;10(1):186. doi: 10.1186/s40478-022-01493-7. Acta Neuropathol Commun. 2022. PMID: 36528671 Free PMC article.
-
Unwanted Exacerbation of the Immune Response in Neurodegenerative Disease: A Time to Review the Impact.Front Cell Neurosci. 2021 Oct 22;15:749595. doi: 10.3389/fncel.2021.749595. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34744633 Free PMC article. Review.
-
APOE ε4 associates with increased risk of severe COVID-19, cerebral microhaemorrhages and post-COVID mental fatigue: a Finnish biobank, autopsy and clinical study.Acta Neuropathol Commun. 2021 Dec 23;9(1):199. doi: 10.1186/s40478-021-01302-7. Acta Neuropathol Commun. 2021. PMID: 34949230 Free PMC article.
References
-
- Dantzer R. Cytokine‐induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

